Method for producing recombinant mixed L-arabinose isomerase
An arabinose and isomerase technology, applied in the biological field, can solve problems such as L-arabinose isomerase has not yet been discovered, affecting the development of D-tagatose industry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
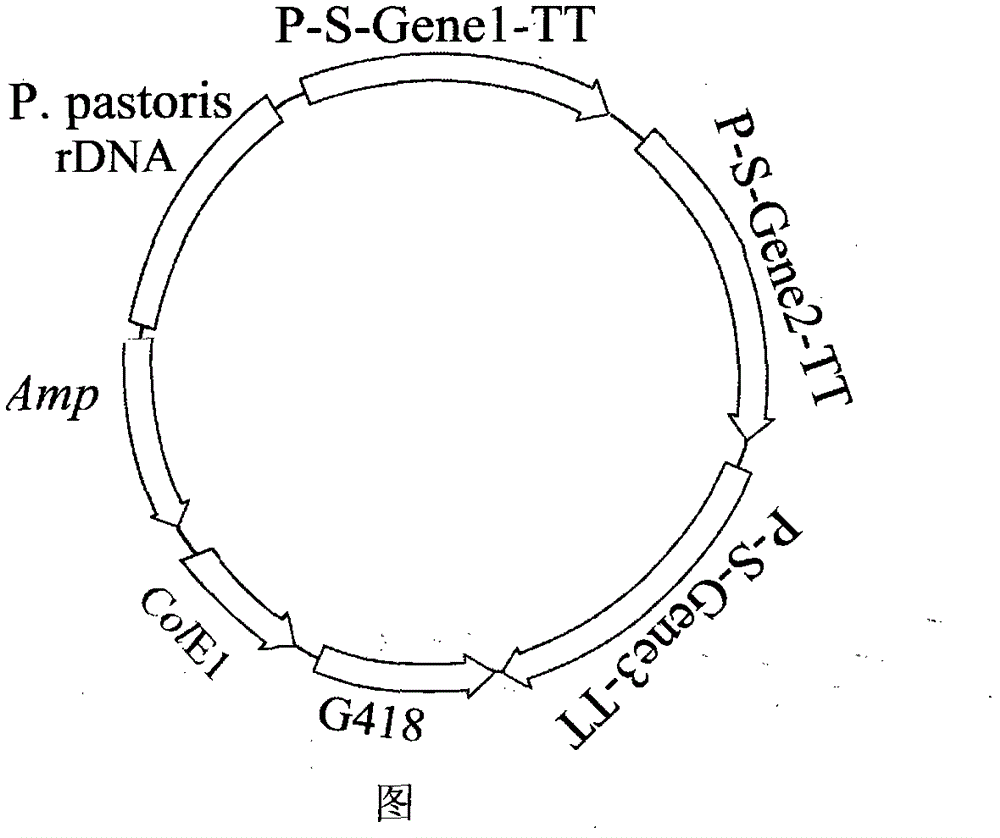
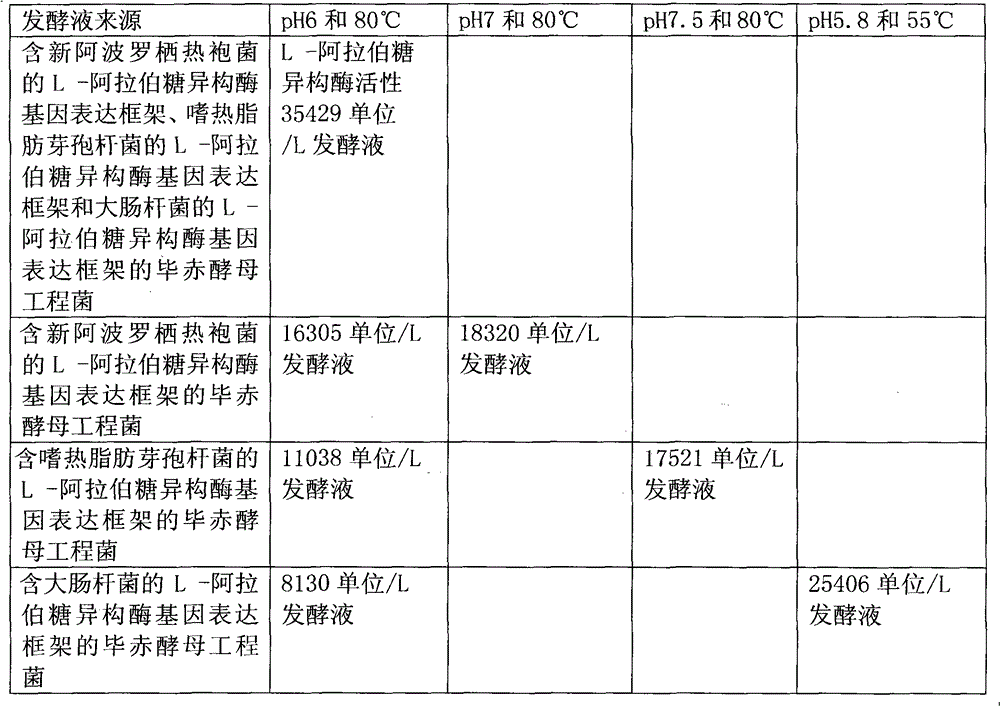
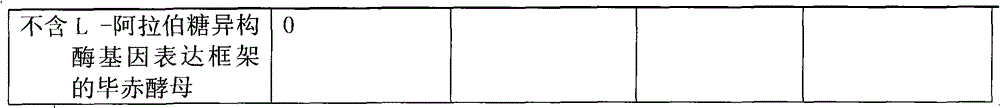
[0019] 1.1 Construction of the L-arabinose isomerase gene expression framework containing Thermotoga neoapollinum, the L-arabinose isomerase gene expression framework of Bacillus stearothermophilus and the L-arabinose isomerase gene of Escherichia coli Expression framework for Pichia expression vector
[0020] 1.1.1 Construction of cloning vector
[0021] A professional DNA sequence synthesis company synthesizes two base complementary double strands containing ampicillin (AMP) gene sequence, polyclonal adapter and E. coli replication origin, and forms cohesive ends at both ends of each DNA strand sequence. It is circularized by the action of DNA ligase to form a DNA cloning vector. The cloning vector was named pPL.
[0022] 1.1.2 Acquiring genes
[0023] ①PCR amplification of the L-arabinose isomerase gene of Thermotoga neoapollianus
[0024] Primer 1:
[0025] 5'cc GAATTC atgatcgatctcaaacagtat3'[Description: The 8 bases of 5' are the restriction enzyme protection bases...
Embodiment 2
[0081] 2.1 Construction of the L-arabinose isomerase gene expression framework containing Thermotoga neoapollinum, the L-arabinose isomerase gene expression framework of Bacillus stearothermophilus and the L-arabinose isomerase gene of Escherichia coli Expression framework for Pichia expression vector
[0082] 2.1.1 Construction of cloning vector
[0083] A professional DNA sequence synthesis company synthesizes two base complementary double strands containing ampicillin (AMP) gene sequence, polyclonal adapter and E. coli replication origin, and forms cohesive ends at both ends of each DNA strand sequence. It is circularized by the action of DNA ligase to form a DNA cloning vector. The cloning vector was named pPL.
[0084] 2.1.2 Acquiring genes
[0085] ①PCR amplification of the L-arabinose isomerase gene of Thermotoga neoapollianus
[0086] Primer 1:
[0087] 5'cc GAATTC atgatcgatctcaaacagtat3'[Description: The 8 bases of 5' are the restriction enzyme protection bases...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com