Preparation and purification of iodixanol
一种碘克沙醇、碘海醇的技术,应用在有机化合物的纯化/分离/稳定化、羧酸酰胺分离/纯化、有机化学等方向,能够解决限制纯化效率和产品质量等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
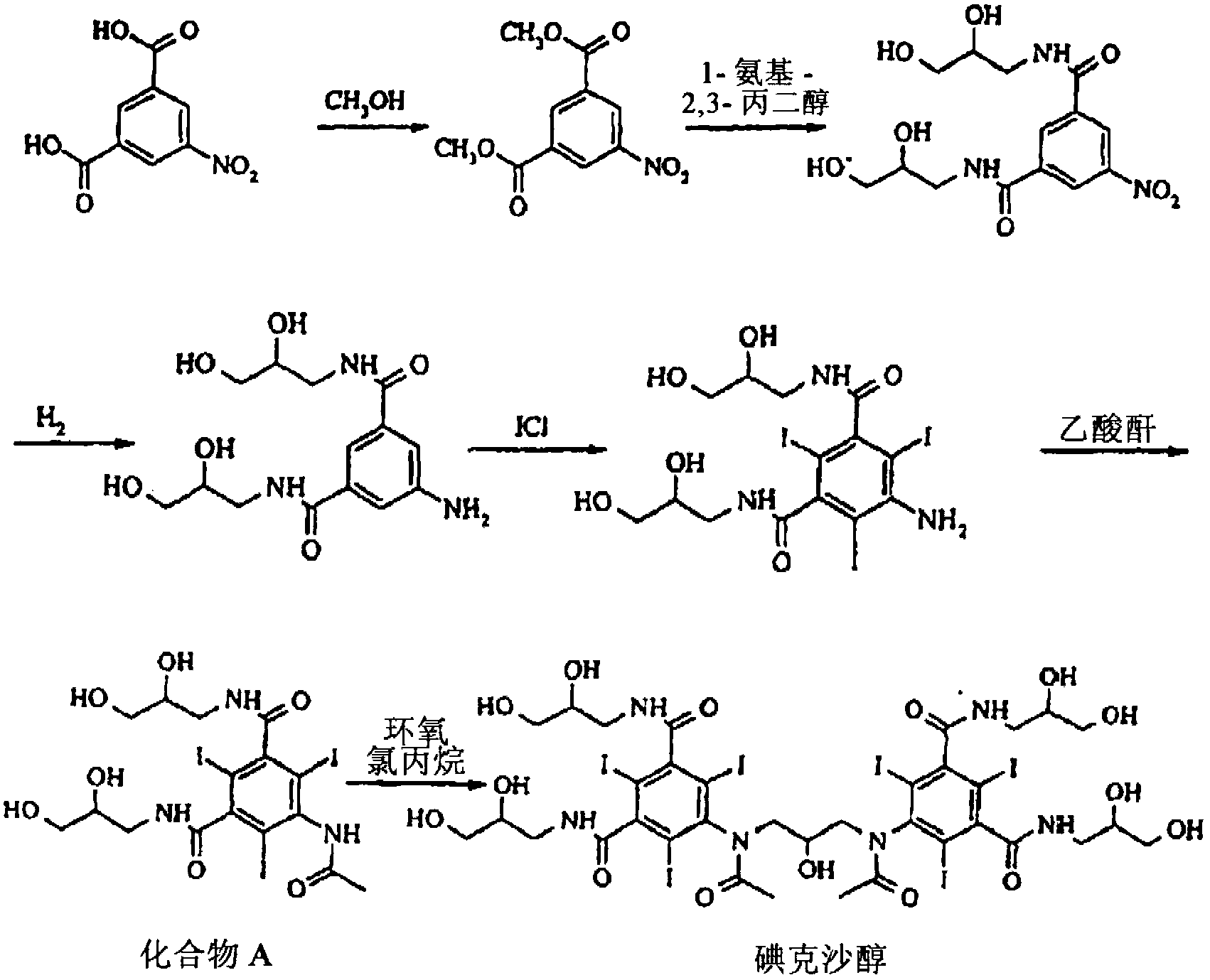
[0088] Compound A (1120.5kg, 1.50kmol) was dissolved in KOH (140.0kg, 2.25kmol) in water (1232.6kg), the temperature was controlled below 20°C, and boric acid (64.9kg, 1.05 kmol), hydrochloric acid (30.4kg, 0.30kmol) was added dropwise, and then epichlorohydrin (83.3kg, 0.90kmol) was added dropwise. The pH during the reaction was 10-11. The reaction was monitored and samples were analyzed by HPLC. When the compound A content was lower than 5%, the reaction was quenched by adding water (1232.6 kg) and adjusting the pH in the range of 5-6 with 18% hydrochloric acid.
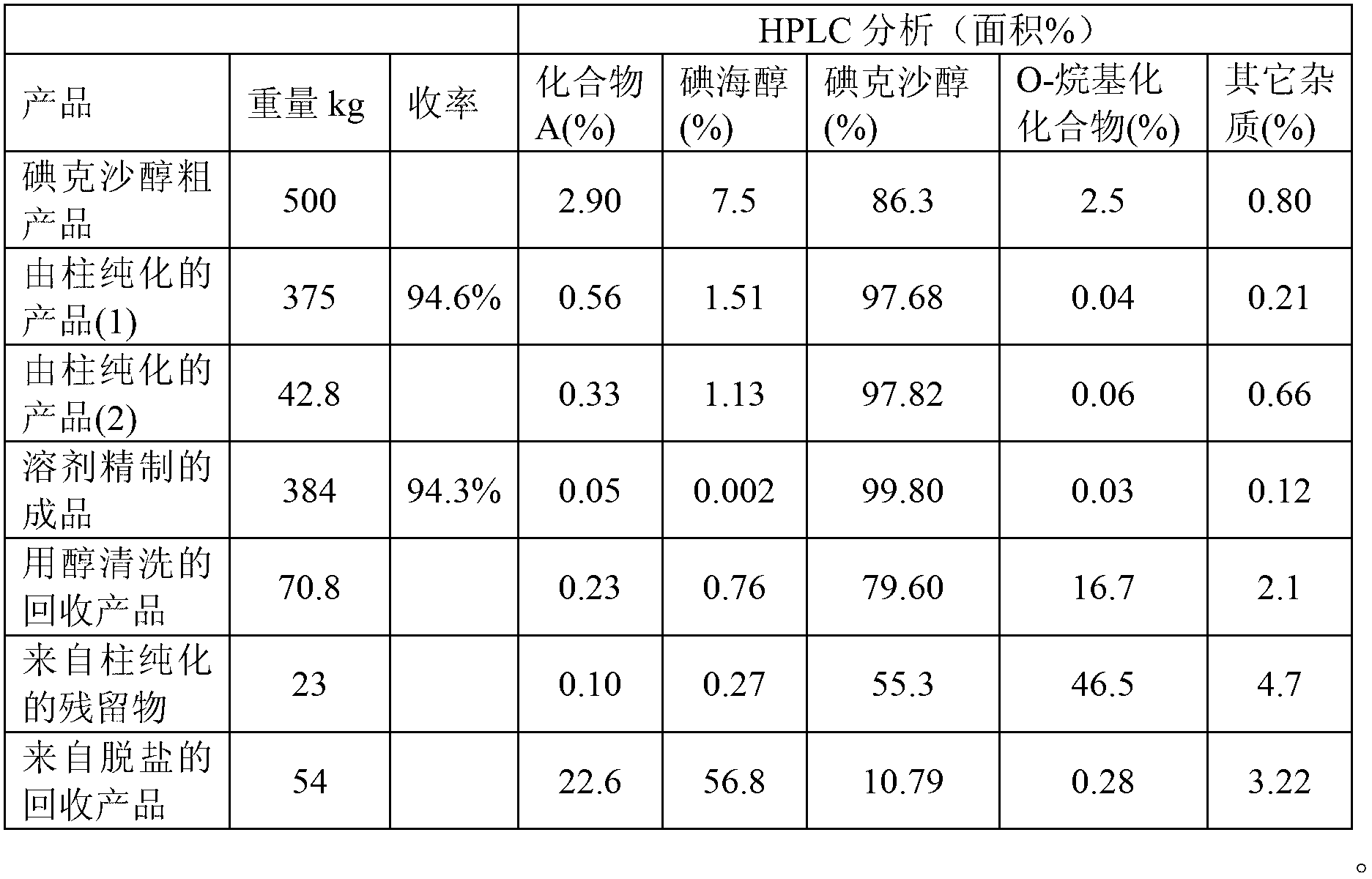
[0089] The reaction mixture was then desalted with active charcoal and filtered. By HPLC analysis, the filtrate contained 86.3% iodixanol, 7.5% iohexol, 2.9% compound A and 2.7% O-alkylated by-products and other impurities.
Embodiment 2
[0091] Compound A (1120.5kg, 1.50kmol) was dissolved in NaOH (78.0kg, 1.95kmol) in water (1232.6kg), the temperature was controlled below 20°C, and boric acid (51.0kg, 0.83 kmol), hydrochloric acid (23.3kg, 0.23kmol) was added dropwise, and then epichlorohydrin (83.3kg, 0.90kmol) was added dropwise. The pH during the reaction was 10-11. The reaction was monitored and samples were analyzed by HPLC. When the compound A content was lower than 5%, the reaction was quenched by adding water (1232.6 kg) and adjusting the pH in the range of 5-6 with 18% hydrochloric acid.
[0092] Inorganic ions were removed by passing through a column containing anion and cation exchange resins, followed by decolorization with activated carbon; the filtrate was concentrated by evaporation to dryness to obtain crude iodixanol (1163.0 kg)
[0093] Contents by HPLC (analysis) were 85.0% iodixanol, 7.1% iohexol, 3.0% compound A, 2.9% O-alkylated by-products and small amounts of other impurities.
Embodiment 3
[0095] Compound A (11.2kg, 15.0mol) was dissolved in NaOH (0.96kg, 24.0mol) in 2-methoxyethanol solution, the temperature was controlled below 20°C, and boric acid (0.65kg, 10.5 mol), hydrochloric acid (0.55kg, 5.4mol) was added dropwise, and then epichlorohydrin (0.75kg, 8.1mol) was added dropwise. The pH during the reaction was 10-11. The reaction was monitored and samples were analyzed by HPLC. When the compound A content was lower than 5%, the reaction was quenched by adding water (12.3 kg) and adjusting the pH in the range of 5-6 with 18% hydrochloric acid.
[0096] The reaction mixture was then decolorized with activated charcoal. By HPLC analysis, the filtrate contained 84.2% iodixanol, 5.1% iohexol, 4.8% compound A and 2.5% O-alkylated by-products and other impurities.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com