Evodiamine dispersion tablets and preparation method thereof
A technology of evodial alkaloid and dispersible tablet, which is applied in the field of evodial alkaloid dispersible tablet and its preparation, and achieves the effects of high dispersion uniformity, favorable absorption and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 The preparation of evodiamine dispersible tablets of the present invention
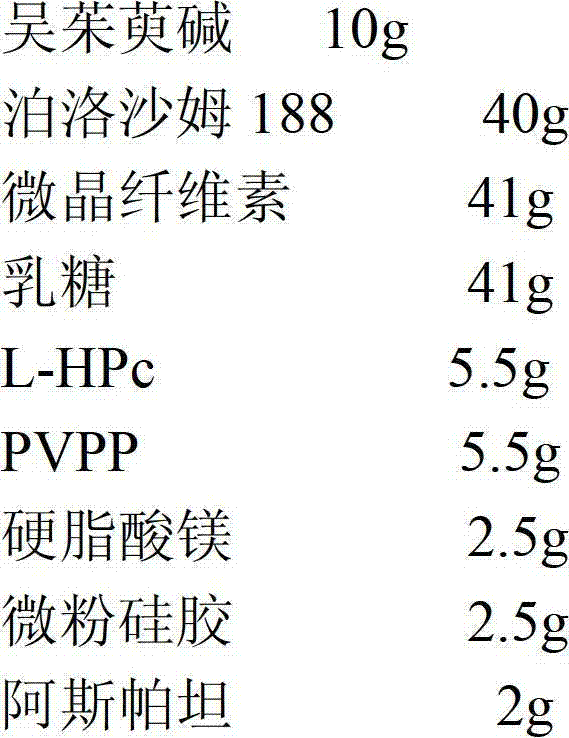
[0028] Evodiamine dispersible tablets include the following weight percentage components to make 1000 tablets
[0029]
[0030] Preparation method: (1) Weigh the raw and auxiliary materials according to the prescription ratio;
[0031] (2) Take evodiamine, grind it to a particle size of 1-10 μm, mix it with poloxamer 188, and prepare a solid dispersion by melting;
[0032] Melting method of the present invention can be carried out according to conventional method, or adopt following operating steps:
[0033] Melt the poloxamer in a constant temperature water bath at 80°C, add evodiamine, stir evenly, pour it out quickly, and move it to -20°C to freeze and solidify, take it out and dry it, crush it, and sieve it for later use.
[0034] (3) Take the solid dispersion prepared in step (2), crush it through an 80-mesh sieve, and mix it evenly with microcrystalline cellulose, lactos...
Embodiment 2
[0036] Embodiment 2 Preparation of evodiamine dispersible tablets of the present invention
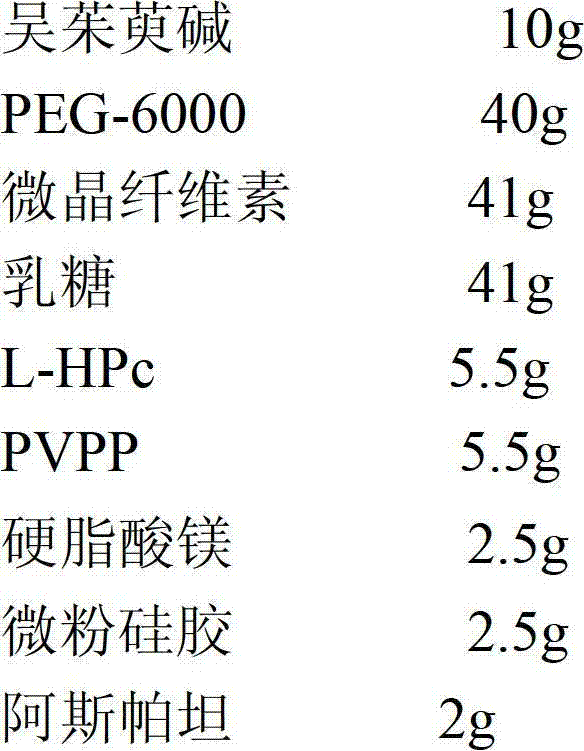
[0037] Evodiamine dispersible tablets include the following weight percentage components to make 1000 tablets
[0038]
[0039] Preparation method: (1) Weigh the raw and auxiliary materials according to the prescription ratio;
[0040] (2) Take evodiamine and grind it to a particle size of 1-10 μm, then mix it with PEG-6000, and prepare a solid dispersion by melting method;
[0041] (3) Take the solid dispersion prepared in step (2), crush it through an 80-mesh sieve, mix it with microcrystalline fiber, lactose, L-HPc, PVPP, aspartame, magnesium stearate and micropowder silica gel, and then directly Tablet
Embodiment 3
[0042] Embodiment 3 Preparation of Evodia Evodia Dispersible Tablets of the present invention
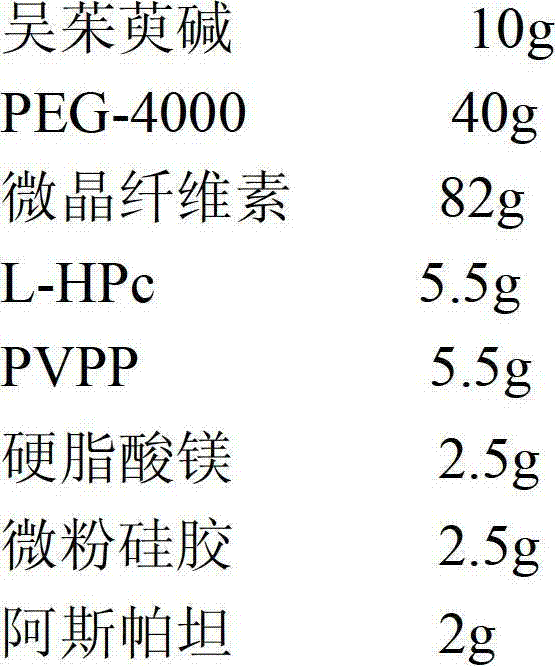
[0043] Evodiamine dispersible tablets include the following weight percentage components to make 1000 tablets
[0044]
[0045] Preparation method: (1) Weigh the raw and auxiliary materials according to the prescription ratio;
[0046] (2) Take evodiamine and grind it to a particle size of 1-10 μm, then mix it with PEG-4000, and prepare a solid dispersion by melting method;
[0047] (3) Take the solid dispersion prepared in step (2), crush it through an 80-mesh sieve, mix it with microcrystalline fiber, L-HPc, PVPP, aspartame, magnesium stearate and micropowder silica gel, and then directly compress it into tablets
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com