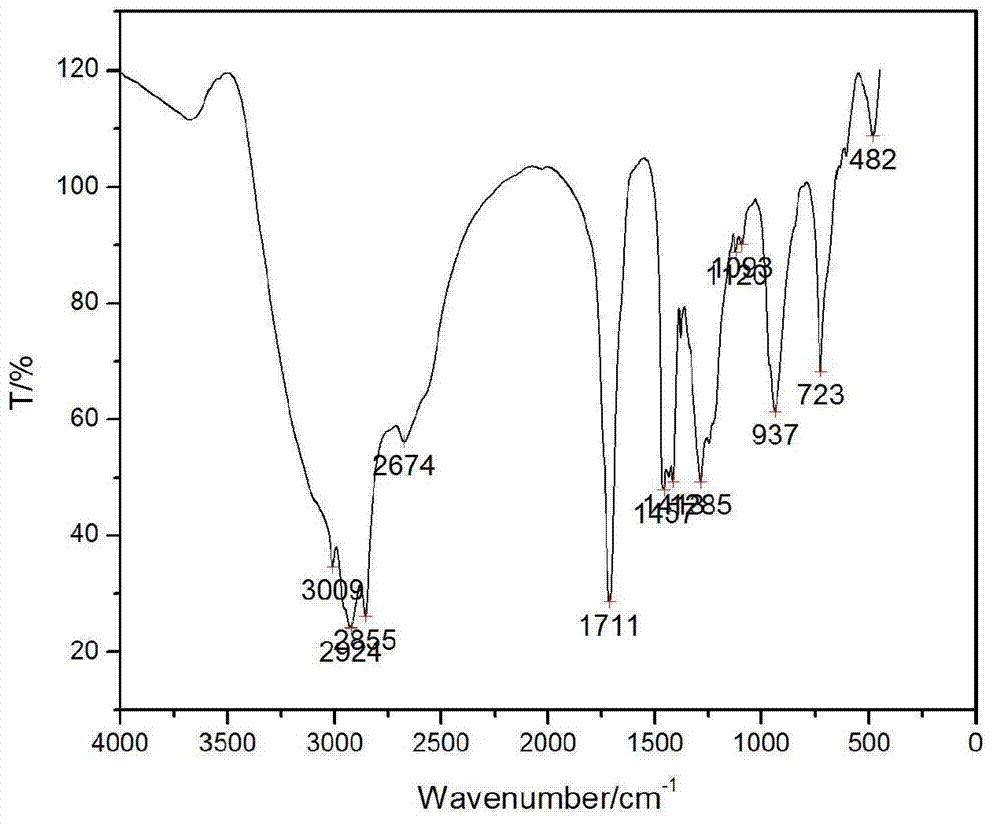
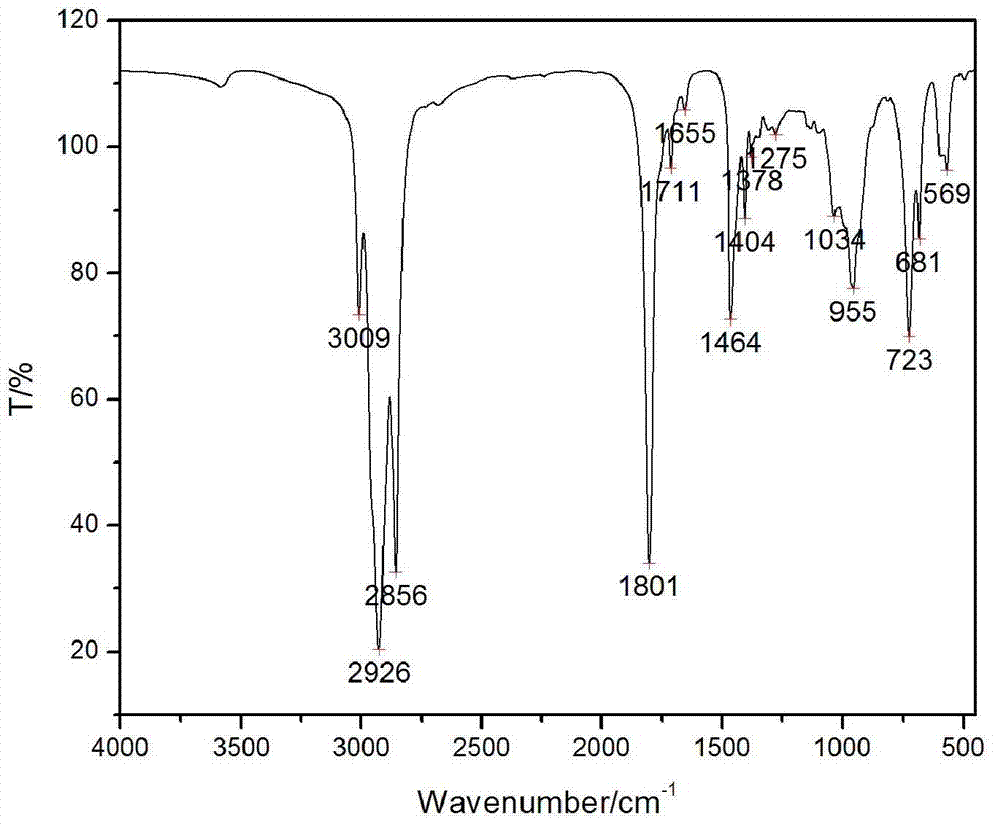
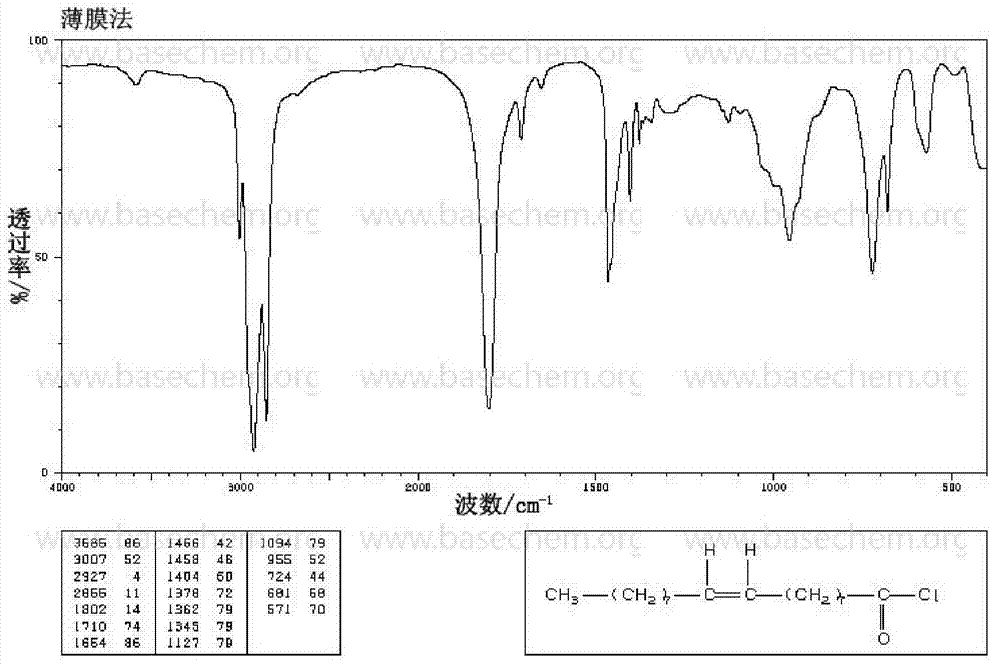
Method for synthesizing oleoyl chloride by triphosgene
A technology of triphosgene and oleoyl chloride, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve problems such as low product purity and yield, environmental pollution, and high requirements for equipment conditions, and achieve high yield And the effect of high yield, safe operation, and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh 0.05mol of purified oleic acid and add it to a 250ml three-necked flask. The temperature of the water bath rises to 60°C and remains constant. Add 0.025mol of triphosgene, and then slowly dropwise add 0.77ml of N,N-dimethylformamide. The constant temperature reaction time is set to 2h, after the reaction is completed, stand for 0.5h~1h to separate the upper layer of the mixture. And calculate the purity and yield of the target product.
[0026] Purity / %
Embodiment 2
[0028] Weigh 0.05mol of purified oleic acid and add it to a 250ml three-necked flask. The temperature of the water bath rises to 60°C and remains constant. Add 0.025mol of triphosgene, and then slowly dropwise add 0.77ml of N,N-dimethylformamide. The constant temperature reaction time is set to 0.5h, after the reaction is completed, stand for 0.5h~1h to separate the upper layer of the mixture. And calculate the purity and yield of the target product.
[0029] Purity / %
Embodiment 3
[0031] 0.05mol of purified oleic acid was weighed into a 250ml three-necked flask, the temperature of the water bath was raised to 80°C and kept constant, 0.025mol of triphosgene was added, and then 0.77ml of N,N-dimethylformamide was slowly added dropwise, and the reaction time at constant temperature was 0.5 h, after the reaction is completed, stand for 0.5h~1h to separate the upper layer of the mixture. And calculate the purity and yield of the target product.
[0032] Purity / %
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com