Antitumor compound, its preparation method and application
A technology for anti-tumor drugs and compounds, applied in the field of medicine, can solve the problems of obvious adverse reactions, affecting the quality of life of patients, and poor selectivity of cytotoxic drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
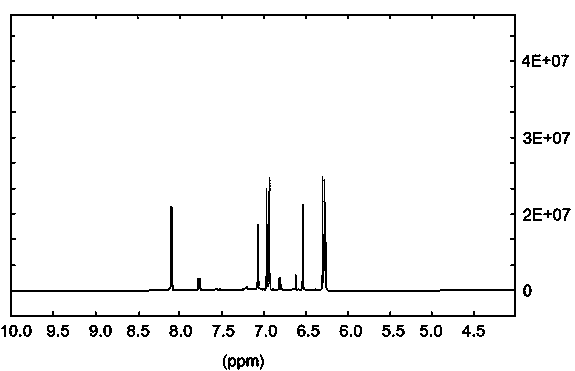
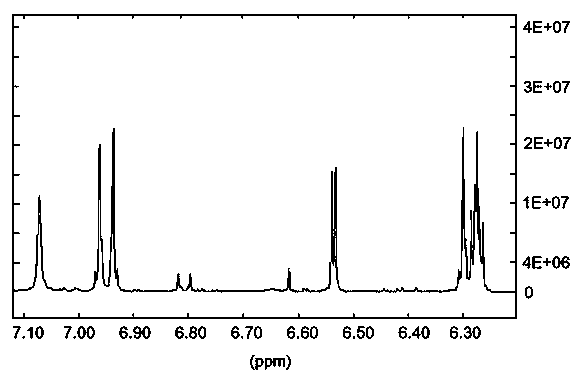
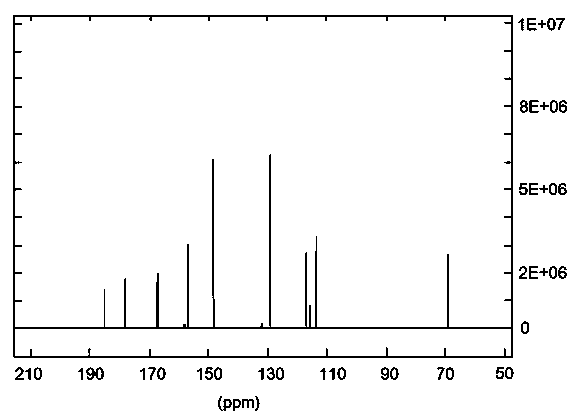
Image
Examples
Embodiment 1
[0099] Preparation of 2-(1-hydroxy-4-one-2,5-cyclohexadiene)-pyran-4-one
[0100] (1) 2-(2-bromoethyl)-1,3-dioxolane and sodium nitrite were nitrated and purified to obtain a yellow liquid substance. The specific operation is as follows: 1 equivalent of 2- (2-Bromoethyl)-1,3-dioxolane, 1.3 equivalents of sodium nitrite, and 0.5 equivalents of phloroglucinol were dissolved in DMSO, mixed uniformly, and reacted at room temperature for 18 hours. The reaction mixture was dispersed in water, extracted twice with an equal volume of dichloromethane, the dichloromethane layer was taken, and the solvent was recovered at 70°C. The product was developed in an iodine tank under TLC conditions with a volume ratio of petroleum ether / acetone of 10:1, and the Rf value was 0.3. Using petroleum ether / acetone with a volume ratio of 10:1 as normal phase silica gel for column chromatography purification to obtain a yellow liquid product, which is judged to be 2-(2-nitroethyl)-1,3- Dioxolane [See...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com