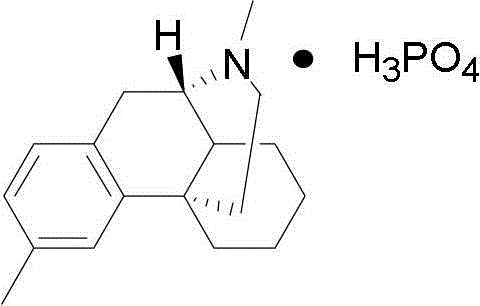
Dimemorfan phosphate crystal form II and preparation method thereof, and pharmaceutical composition
A technology for dimethylorphinyl phosphate and its composition, which is applied in the field of dimethylorphinyl phosphate crystal form II and its preparation and pharmaceutical composition, can solve problems such as the description of recrystallization methods, achieve excellent purity, excellent reproducibility, Effects of good pharmaceutical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The preferred preparation method of the present invention is: add 20 g of the crude product of dimethylorphinyl phosphate into 200-2000 ml of methanol, heat to reflux to dissolve it, filter, place the filtrate at room temperature for 1-20 h to crystallize, filter, and reduce the temperature below 60 ° C. Dry under pressure to obtain a white solid, dimethylmorphan phosphate crystal form II.
[0051] A dimethylmorphan phosphate crystal form II prepared by the above-mentioned preparation method, which is characterized by the following X-ray diffractometer diagram, and represents the relative relative to the percentage of the strongest line with the interplanar distance d and the Bragg angle 2θ. X-ray powder diffraction pattern expressed by intensity:
[0052] 2θ angle data d-value data Intensity data (%) 5.5 16.1 100.0 11.0 8.0 2.7 12.2 7.3 4.6 12.8 6.9 3.1 13.6 6.5 6.2 14.3 6.2 3.7 ...
Embodiment 1
[0056] Add 20g of dimethylorphinyl phosphate and 80ml of anhydrous methanol into the flask, heat to reflux, dissolve under stirring, cool to room temperature after dissolving, stir at room temperature for 3h, filter, wash with appropriate amount of cold anhydrous methanol, and depressurize at 50°C After drying, 16 g of white solid was obtained, which is a new crystal form of dimethylorphinyl phosphate (form II).
[0057] 1H-NMR (D 2 O, ppm): 2.198~2.223(d,1H), 2.306(s,3H), 2.865(s,3H), 3.530(s,1H), 7.022~7.038(d,1H), 7.095~7.110(d, 1H), 7.159 (s, 1H). ESI-MS: 256.2.
Embodiment 2
[0059] Take 20g of dimethylorphinyl phosphate, add 150ml of ethanol, heat to reflux to dissolve, filter while hot, keep the filtrate at room temperature, and stir at room temperature for 8h. Filter and dry under reduced pressure at 50° C. to obtain 14 g of white solid, which is a new crystal form of dimethylorphane phosphate (crystal form II).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com