Preparation method of 4AA diastereoisomer
A technology of epimerization and compound, applied in the field of preparation of 4AA diastereoisomers, can solve the problems of difficult preparation and purification, poor selectivity, inability to synthesize 4AA diastereoisomers in a targeted manner, and achieve simple purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
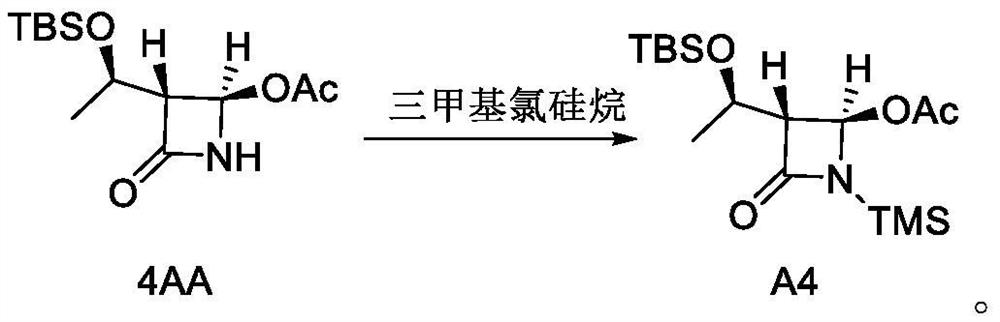
[0059] Synthesis of Example 1 Compound A4
[0060]
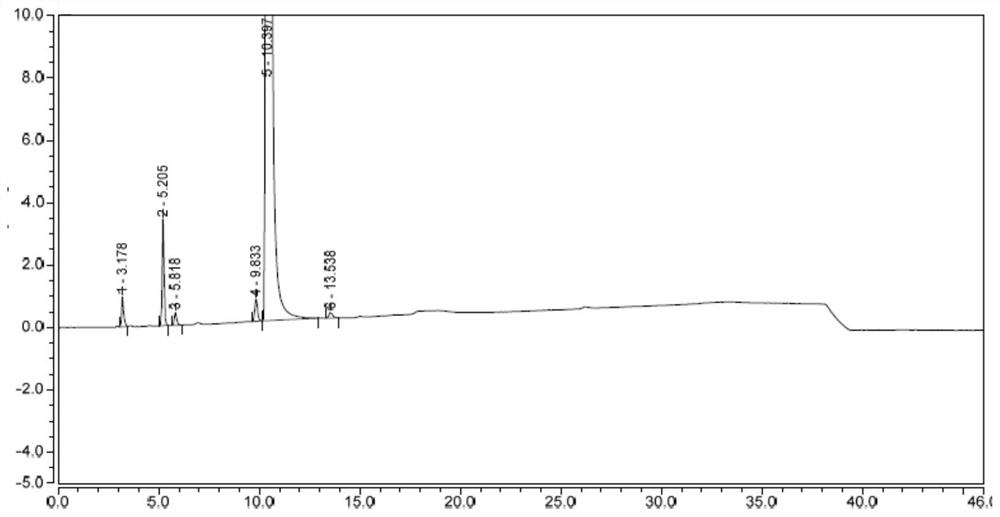
[0061] 300 g of tetrahydrofuran, 28.7 g of 4AA and 15 g of triethylamine were added to a 500 mL three-necked flask at 20°C. The system was cooled to 0-10°C, 30 g of trimethylchlorosilane was added dropwise, the dropping was completed, the temperature of the system was raised to 40°C, and the reaction was continued for 2 hours. After TLC showed that the raw material 4AA was completely consumed, the system was cooled to 20° C. and concentrated to remove tetrahydrofuran to obtain crude A4, which was purified by column chromatography once to obtain 21.8 g of pure A4 with a yield of 60.5% and a purity of 97.9%.
Embodiment 2
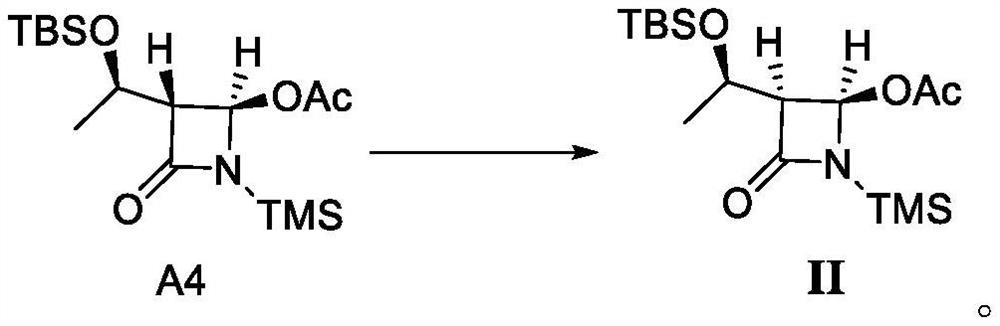
[0062] Embodiment 2 is as the synthesis of compound shown in formula I
[0063]
[0064] 200 g of anhydrous tetrahydrofuran (water 0.01% or less) and 10 g of compound A4 were added to a 500 mL three-neck flask at 20°C. The system was cooled to -78 °C, and 50 mL of lithium hexamethyldisilazide (LiHMDS) (2M, n-hexane) was added dropwise. After the dropwise addition was completed, the system was heated to 20 °C within 2 hours and kept at the temperature for 1 hour. The mixed solution of the compound shown in II was detected by HPLC, and the reaction system was quenched into 100 g of ice water (containing 5.0 g of acetic acid). The system was concentrated under reduced pressure, and 100 g of ethyl acetate and 50 g of ice water were added to the concentrated residue to separate the layers; the lower aqueous phase was extracted once with 20 g of ethyl acetate, and the organic phases were combined and washed once with 50 g of saturated aqueous sodium bicarbonate solution. The org...
Embodiment 3
[0072] Embodiment 3 is as the synthesis of compound shown in formula I
[0073]
[0074] 200 g of anhydrous n-hexane (water content of 0.01% or less) and 11.2 g of compound A4 were added to a 500 mL three-necked flask at 20°C. The system was cooled to -78°C, and 50 mL of lithium diisopropylamide (LDA) (2M, n-hexane) was added dropwise. The dropwise addition was completed. The mixed solution of the indicated compounds was detected by HPLC. The reaction system was quenched into 100 g of ice water and stirred at 20° C. for 1 h (containing 5.0 g of acetic acid). The system was concentrated under reduced pressure, and 100 g of ethyl acetate and 50 g of ice water were added to the concentrated residue to separate the layers; the lower aqueous phase was extracted once with 20 g of ethyl acetate, and the organic phases were combined and washed once with 50 g of saturated aqueous sodium bicarbonate solution. The organic phase was concentrated and dried to obtain a crude product, wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com