Preparation method for lurasidone hydrochloride
A technology of lurasidone hydrochloride and hydrochloric acid is applied in the preparation field of lurasidone hydrochloride, which can solve the problems of low acetone and the like, and achieve the effects of improving durability, high yield, and improving stability and reliability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
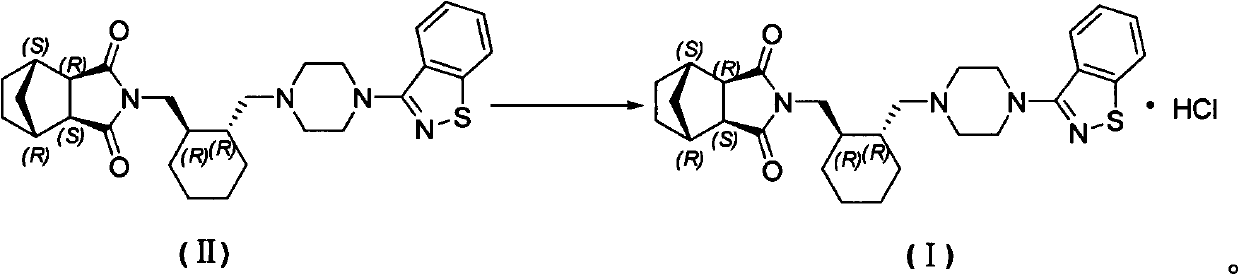
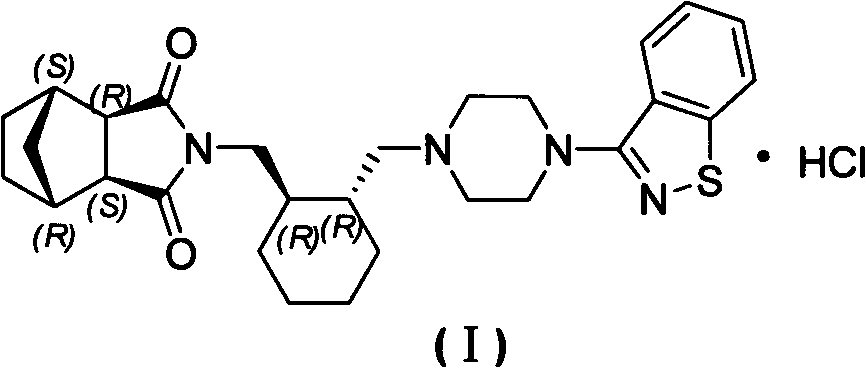
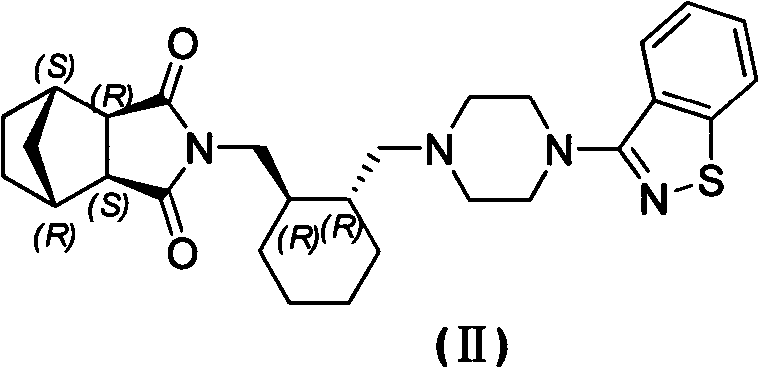
[0025] (3aR, 4S, 7R, 7aS)-2-[(1R, 2R)-2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl]cyclohexyl Methyl]hexahydro-1H-4,7-methylisoindole 1,3-dione (Compound II) (5 g) was dissolved in acetone (53 g) with heating. Under reflux state, dropwise add the acetone (12g) solution of 36% hydrochloric acid (1.13g), reflux and stir for 2h after dropwise addition, after cooling to room temperature, filter, obtain lurasidone hydrochloride (5.16g) after vacuum drying at 60°C. g, yield 96%): HPLC purity 99.86%, the largest single impurity 0.06%, acetone residue 0.13%.
Embodiment 2
[0027] (3aR, 4S, 7R, 7aS)-2-[(1R, 2R)-2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl]cyclohexyl Methyl]hexahydro-1H-4,7-methylisoindole 1,3-dione (compound II) (2 g) was heated and dissolved in acetone (21.2 g). Under reflux state, dropwise add the acetone (6.5g) solution of 18% hydrochloric acid (0.91g), reflux and stir 2h after dropwise addition, after cooling to room temperature, filter, obtain lurasidone hydrochloride ( 2.14 g, yield 99%): HPLC purity 99.83%, the maximum simplex 0.06%, acetone residue 0.21%.
Embodiment 3
[0029] (3aR, 4S, 7R, 7aS)-2-[(1R, 2R)-2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl]cyclohexyl Methyl]hexahydro-1H-4,7-methylisoindole 1,3-dione (compound II) (2 g) was heated and dissolved in acetone (21.2 g). Under reflux, add 14.4% hydrochloric acid (1.13g) in acetone (6.5g) dropwise. After the dropwise addition, stir at the same temperature for 2 hours. After cooling to room temperature, filter, and vacuum dry at 60°C to obtain lurazil hydrochloride Ketone (1.95 g, yield 95%): HPLC purity 99.83%, maximum simplex 0.07%, residual acetone 0.051%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com