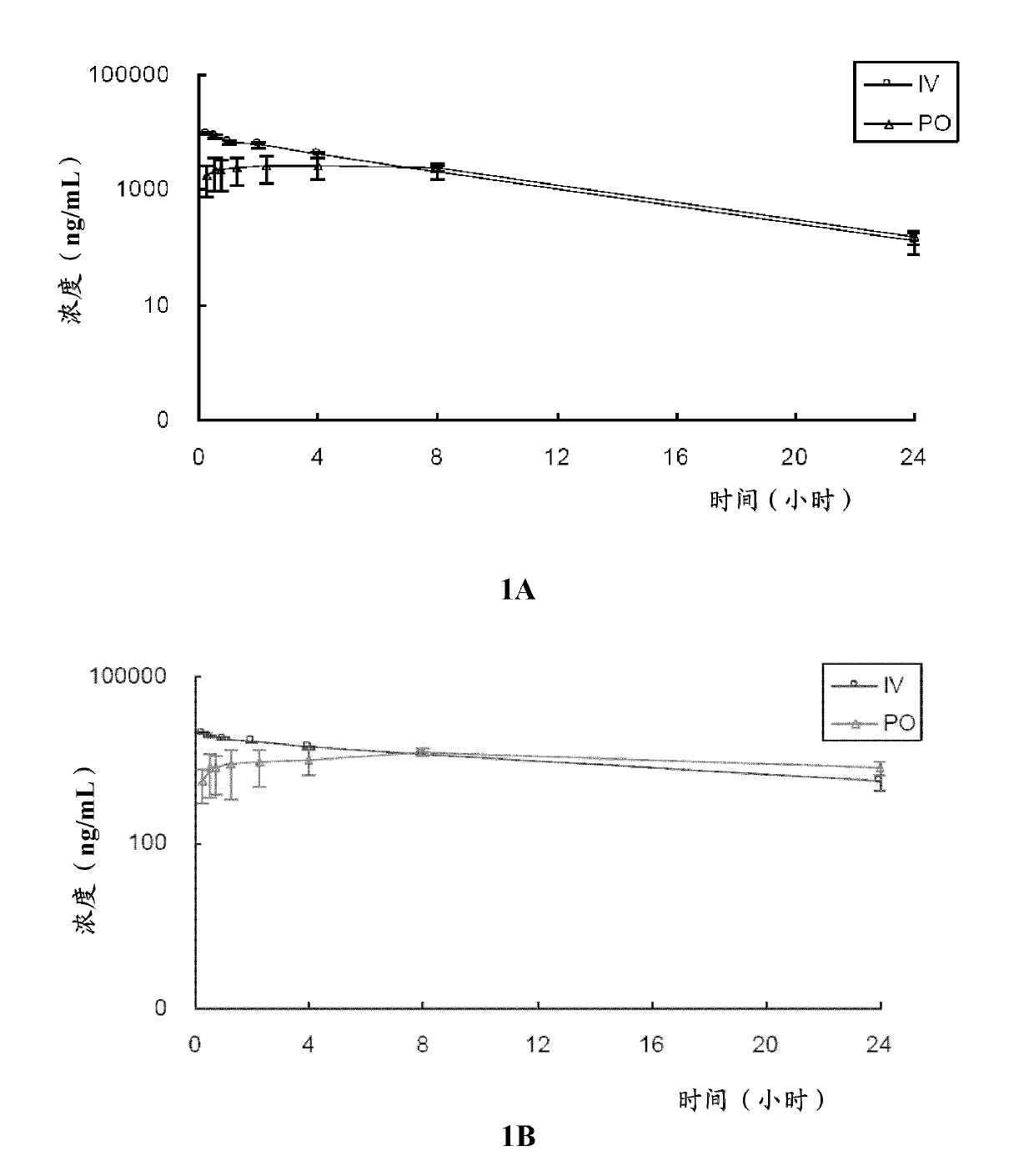
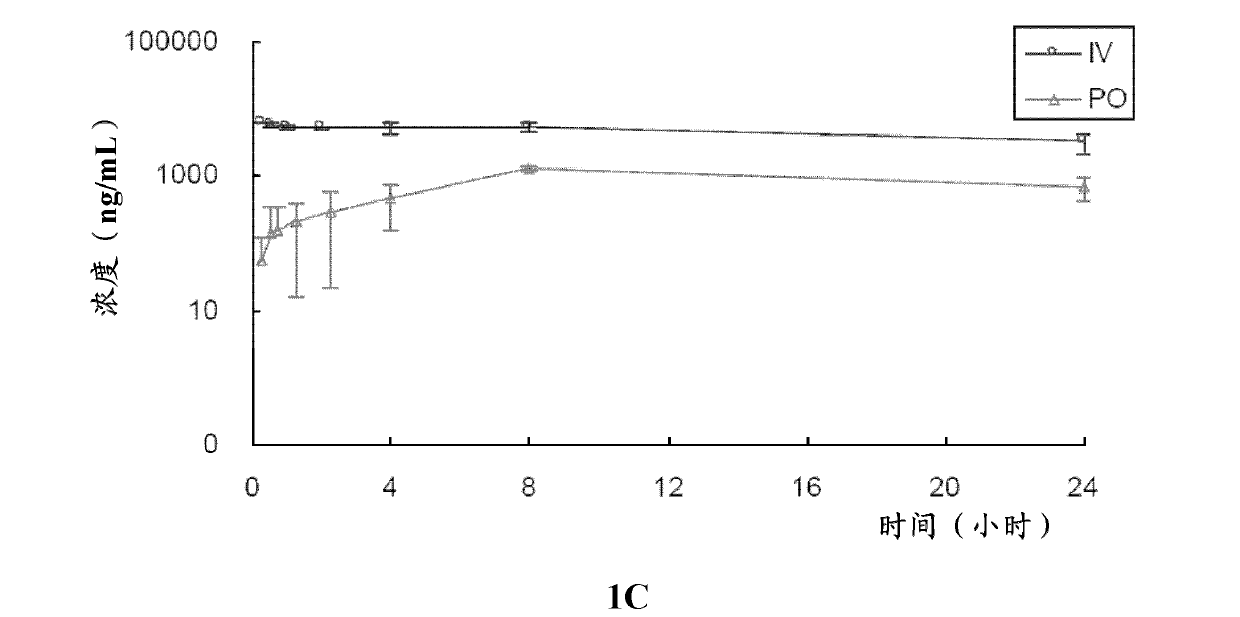
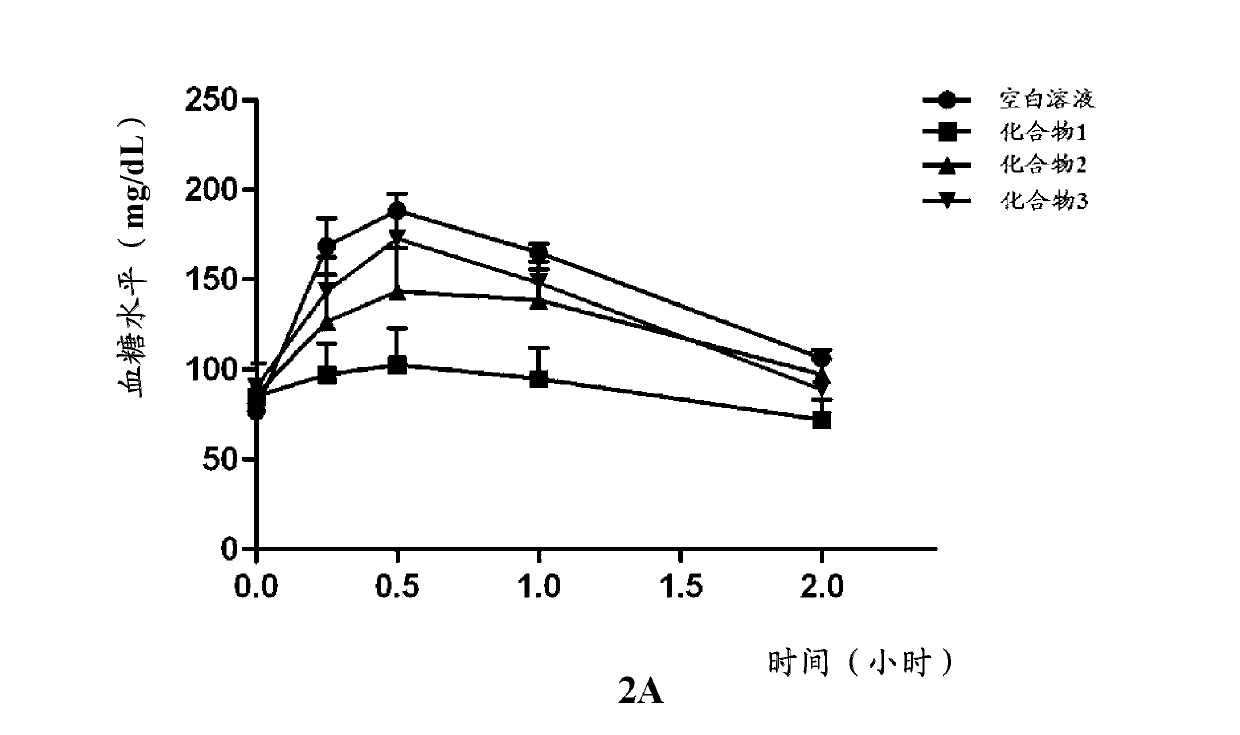
Inhibitor of sodium-dependent glucose transport protein and preparation method therefor and use thereof
A technology of solvates and compounds, applied in the field of medicine, to achieve the effects of high yield, more absorption, and long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Embodiment 1: the preparation of compound 2 of the present invention
[0122]
[0123] step 1):
[0124]
[0125] At -7°C, (3R, 4S, 5S, 6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-one (14.2g) and N - To a solution of methylmorpholine (80 mL) in dry THF (120 mL) was added TMSCl (71 mL), then the mixture was slowly warmed to room temperature and stirred overnight. At 10 ° C, toluene (160 mL) and H 2 O (300 mL) was poured into the mixture, then washed with H 2 The organic phase was washed with O (120 mL), 1M HCl (150 mL x 4) and brine (120 mL). Na 2 SO 4 It was dried and then concentrated to give crude product which was purified by silica gel column chromatography (PE / EA=10 / 1). 30 g of (3R,4S,5R,6R)-3,4,5-tris(trimethylsilyloxy)-6-((trimethylsilyloxy)methyl)tetrahydro-2H-pyran were obtained -2-one.
[0126] Step (2):
[0127]
[0128] To a solution of 5-bromo-2-chlorobenzoic acid (40 g) in DCM (300 mL) was added dropwise (COCl) at room t...
Embodiment 2
[0153] Embodiment 2: the preparation of compound 3 of the present invention
[0154]
[0155] step 1):
[0156]
[0157] With embodiment 1 step (1).
[0158] Step (2):
[0159]
[0160] With embodiment 1 step (2).
[0161] Step (3):
[0162]
[0163] With embodiment 1 step (3).
[0164] Step (4)-1:
[0165]
[0166] To a solution of (5-bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone in dry DCM was slowly added BBr at -78 °C 3 (4.4g, 3eq), then the mixture was stirred at 0°C for 1 h and 24g at room temperature. TLC (PE / EA=1 / 1) showed the starting material was completely reacted. Slowly add saturated NaHCO to the mixture 3 , to adjust the pH to 8-9. The mixture was then extracted with DCM, the combined organic phases were washed with Na 2 SO 4 Dry and concentrate. The crude product (2.5 g) was used directly in the next step.
[0167] Step (4)-2:
[0168]
[0169] To a solution of (5-bromo-2-chlorophenyl)(4-hydroxyphenyl)methanone (6.2 g) in dry DMF...
Embodiment 3
[0184] Embodiment 3: in vitro stability test
[0185] In this example, the in vitro metabolic stability of compound 2 and compound 3 of the present invention was tested, and compared with known compound 1.
[0186] Test compounds: compound 1, compound 2 and compound 3;
[0187] Control compound: verapamil.
[0188] Microsomes: Human liver microsomes (HMMC; PL050B) and male rat liver microsomes (RTMC; RT046) were purchased from CellzDirect (Invitrogen); stored at -80°C until use.
[0189] method:
[0190] 1) Prepare the mother solution as shown in Table 1, and then add the test compound or control compound, so that the final concentration of these compounds in the reaction system is 2 μM. The mixed solution was then preheated at 37°C for 2 minutes.
[0191] Table 1. Preparation of mother liquors
[0192]
[0193] 2) NADPH was added to the mixed solution to make the final concentration 1 mM, and then the reaction system was placed at 37°C. In the blank control, the sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com