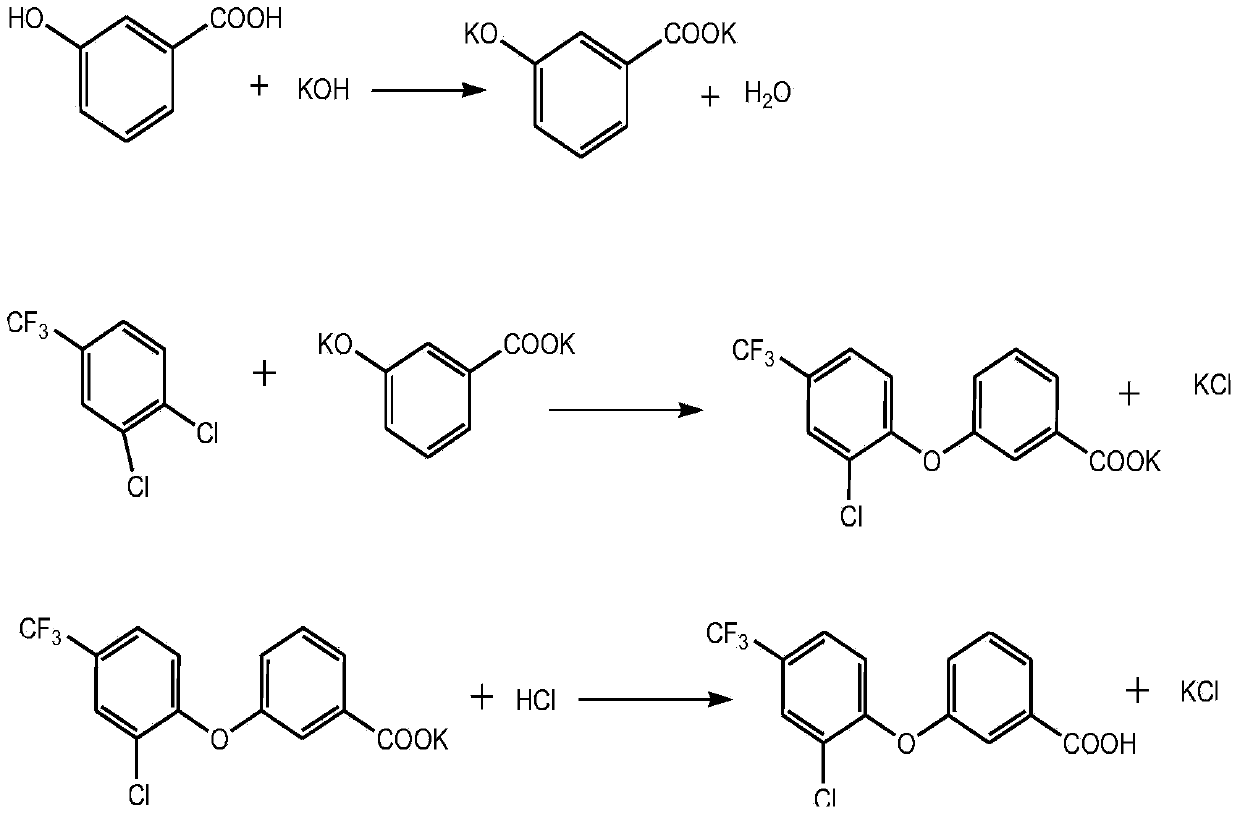
Preparation method for 3- (2-chloro-4- (trifluoromethyl) phenoxy) -benzoic acid
A technology of trifluoromethyl and m-hydroxybenzoic acid, applied in carboxylate preparation, organic chemistry, etc., can solve the problems of low yield and long reaction cycle, and achieve high yield, short reaction cycle and reduced dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 500mL flask, add 60g (0.426mol) of m-hydroxybenzoic acid, 58g (0.936mol) of potassium hydroxide, 3.6g of 15-crown-5, 240g of dimethyl sulfoxide, and 120g of toluene. Slowly add 110.9g (0.511mol) of 3,4-dichlorobenzotrifluoride. After the addition, the temperature is raised to 130-140°C, and the reaction is kept for 6 hours. The residual hydroxybenzoic acid is 0.88%. Add water to dissolve, acidify with hydrochloric acid to pH = 1, filter and dry to obtain 136.3g (0.410mol) of product 3-(2-chloro-4-(trifluoromethyl)phenoxy)-benzoic acid, content 95.1%, yield The rate is 96.3%.
Embodiment 2
[0022] In a 500mL flask, add 60g (0.426mol) of m-hydroxybenzoic acid, 54g (0.871mol) of potassium hydroxide, 2.4g of 18-crown-6, 180g of dimethyl sulfoxide, and 200g of toluene. Slowly add 101.7g (0.468mol) of 3,4-dichlorobenzotrifluoride. After the addition, the temperature is raised to 135-150°C, and the reaction is kept for 6.5 hours. The residue of hydroxybenzoic acid reaches 0.95%. After adding water to dissolve, acidify with hydrochloric acid to pH = 1, and finally filter and dry to obtain the product 3-(2-chloro-4-(trifluoromethyl)phenoxy)-benzoic acid 137.5g (0.416mol), content 95.5% , yield 97.5%.
Embodiment 3
[0024] In a 500mL flask, add 60g (0.426mol) m-hydroxybenzoic acid, 60.6g (0.98mol) potassium hydroxide, 200g dimethyl sulfoxide, 180g toluene and stir, add 3.0g 18-crown-6 in batches within 1 hour, add After completion, heat up and reflux until no water is taken out. Slowly add 106.3g (0.489mol) of 3,4-dichlorobenzotrifluoride. After the addition, the temperature is raised to 135-155°C, and the reaction is kept for 6 hours. The residue of hydroxybenzoic acid reaches 0.93%. After adding water to dissolve, acidify with hydrochloric acid to pH = 1, and finally filter and dry to obtain the product 3-(2-chloro-4-(trifluoromethyl)phenoxy)-benzoic acid 138.5g (0.417mol), content 95.1% , yield 97.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com