Methods for synthesizing molybdopterin precursor z derivatives
A compound and reaction technology, applied in the field of synthesizing molybdenum pterin precursor Z derivatives, can solve problems such as limited availability of therapeutically active agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0256] The preparation of the compounds provided herein may involve the protection and deprotection of various chemical groups. One skilled in the art can readily determine the need for protection and deprotection and select appropriate protecting groups. The chemistry of protecting groups can be found, for example, in Protecting Group Chemistry, 1 st Ed., Oxford University Press, 2000; March's Advanced Organic chemistry: Reactions, Mechanisms, and Structure, 5 th Ed., Wiley-Interscience Publication, 2001; and Peturssion, S.et al., "Protecting Groups in Carbohydrate Chemistry," J.Chem.Educ., 74 (11), 1297 (1997), the respective full The contents are incorporated into this application by reference.
[0257] The reaction can be monitored according to any suitable method known in the art. For example, product formation can be achieved by spectroscopic methods such as nuclear magnetic resonance spectroscopy (e.g. 1 H or 13 C), infrared spectroscopy, spectrophotometry (e.g....
Embodiment 1
[0712] Example 1. Preparation of Precursor Z (cPMP)
[0713] Program 20
[0714]
[0715]
[0716] test
[0717] Air-sensitive reactions were performed under argon. The organic solution was treated with anhydrous MgSO 4 Dry, and the solvent is evaporated under reduced pressure. Anhydrous and chromatographic solvents were obtained commercially (anhydrous grade solvents were purchased from Sigma-Aldrich Fine Chemicals) and were used without any further purification. Thin layer chromatography (t.l.c.) was coated with 60F 254 Silicone on glass or on aluminum sheets. Organic compounds were visualized as follows: under UV light or using ammonium molybdate (5 wt %) and cerium(IV) sulfate 4H 2 O (0.2wt%) in H 2 SO 4 (2M) solution in aqueous solution, I 2 (0.2%) and one of KI (7%) in H 2 SO 4 Impregnation of solutions in (1M) or 0.1% ninhydrin in EtOH. Chromatography (flash column) was performed on silica gel (40-63 [mu]m) or on an automated system with a continuous g...
Embodiment 2
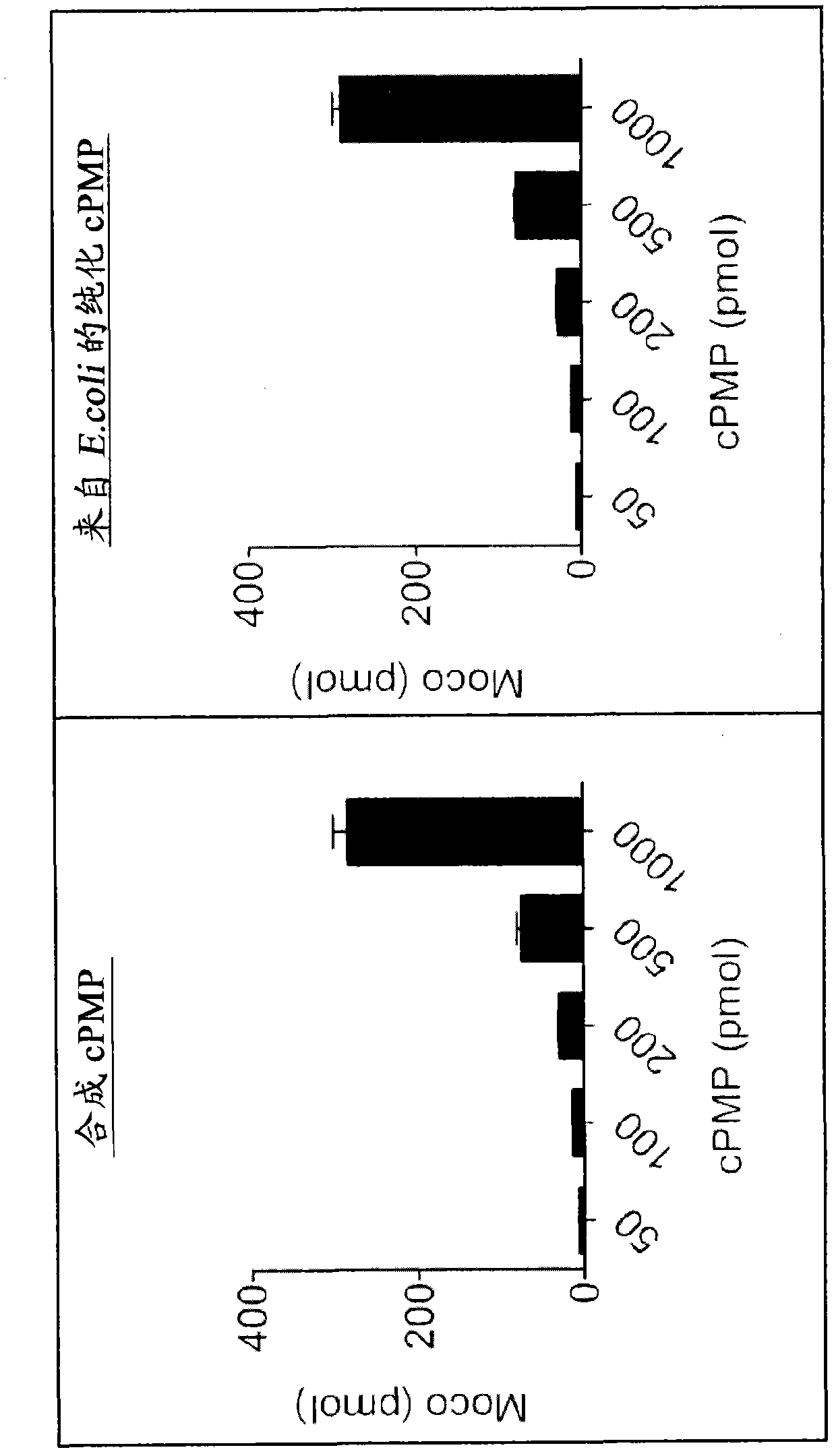
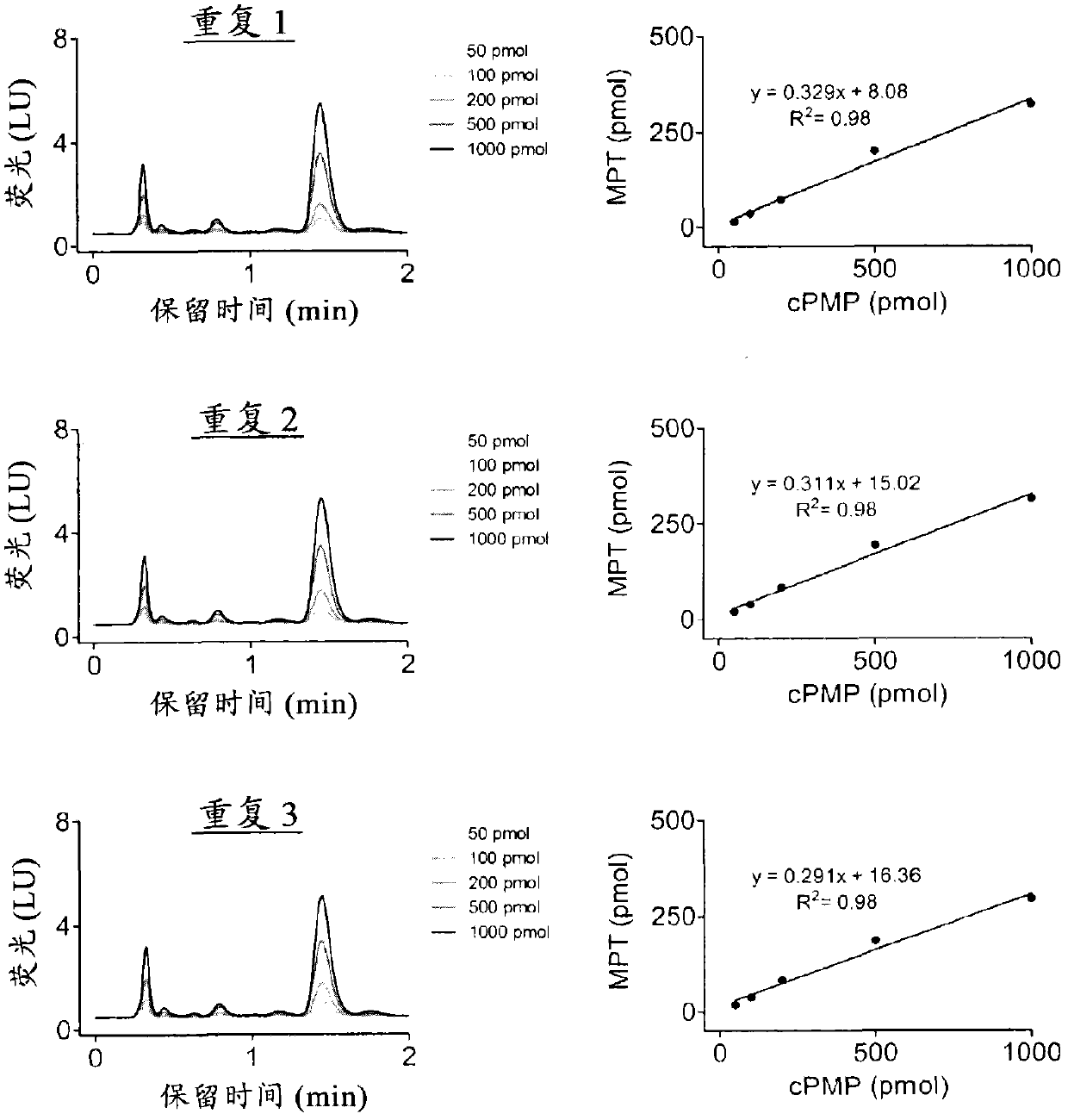
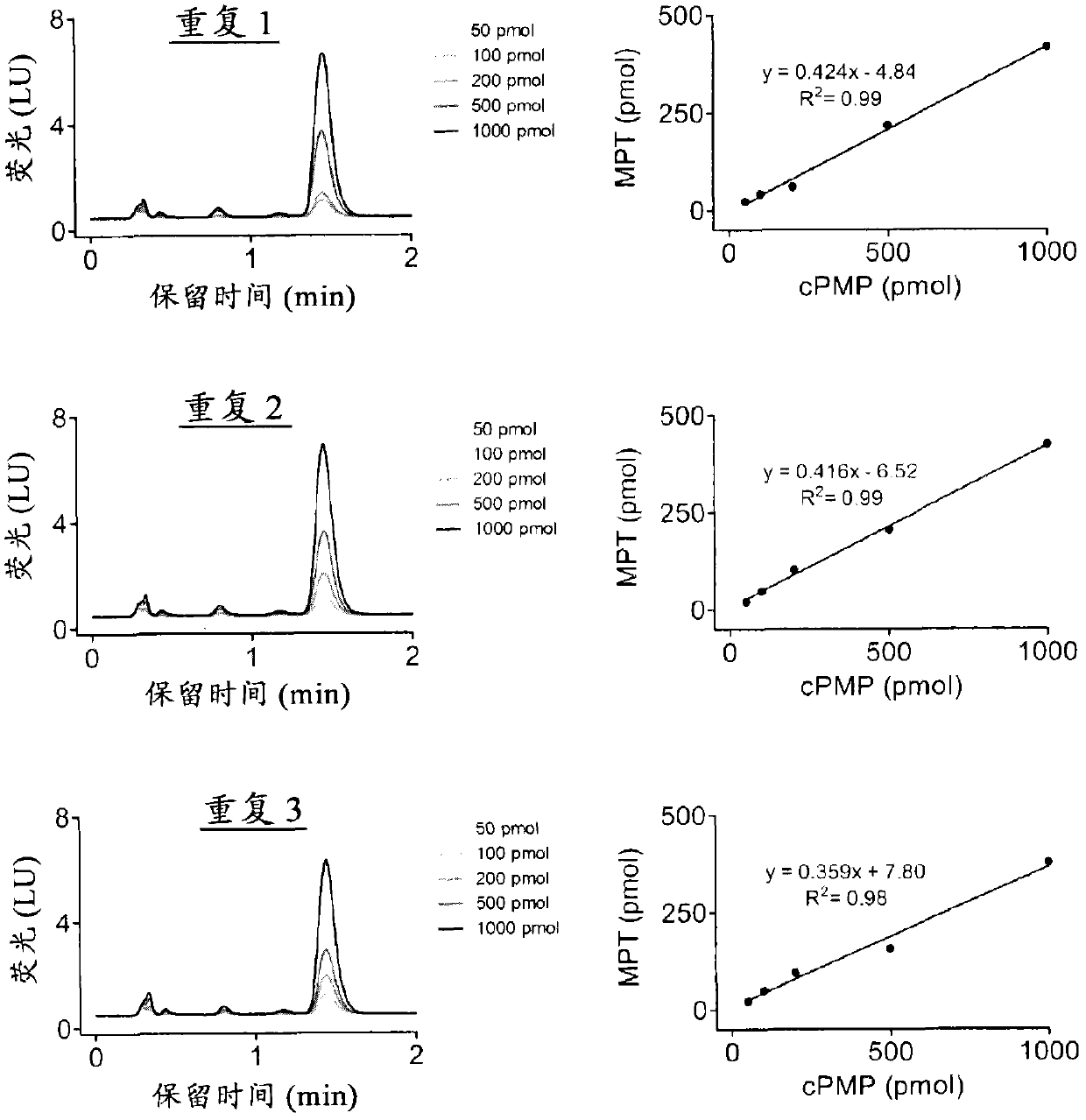
[0731] Example 2. Comparison of Precursor Z(cPMP) prepared synthetically and Precursor Z(cPMP) prepared by E. coli in the in vitro synthesis of Moco
[0732] The in vitro synthesis of Moco was compared using samples of the synthetic precursor Z (cPMP) and cPMP purified from E. coli. Moco synthesis also included the use of purified components E. coli MPT synthase, bridging protein, molybdate, ATP, and apo-sulfite oxidase. See US Patent 7,504,095; and "Biosynthesis and molecular biology of the molybdenum cofactor (Moco)" in Metal Ions in Biological Systems, Mendel, Ralf R. and Schwarz, Günter, Informa Plc, 2002, Vol. 39, pages 317-68. The assay is based on conversion of cPMP to MPT, subsequent molybdate insertion using recombinant bridging proteins and ATP and finally reconstitution of human apo-sulfite oxidase.
[0733] Such as figure 1 As shown, Moco synthesis from synthetic cPMP was confirmed, and no difference in Moco transformation was found compared to E. coli purified c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com