Kinetic resolution method of chiral amine
A kinetic separation, chiral amine technology, applied in fermentation and other directions, can solve the problems of high environmental pollution, low optical purity of products, low separation yield, etc., and achieves improved performance, great application value, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
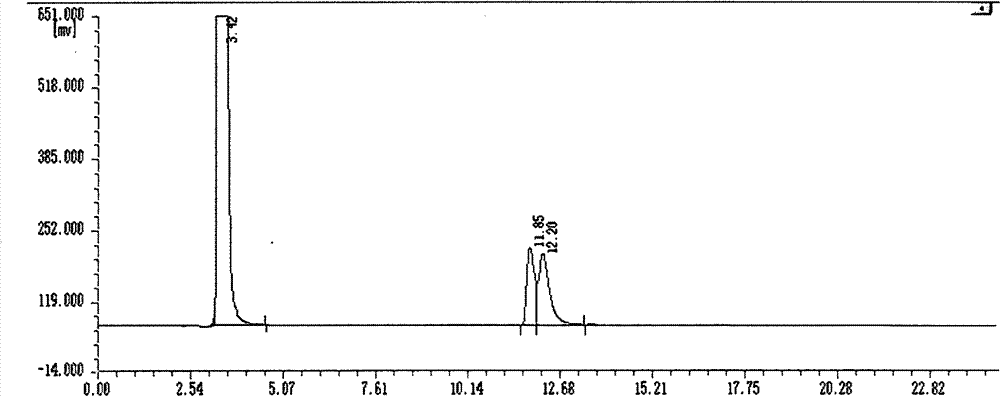
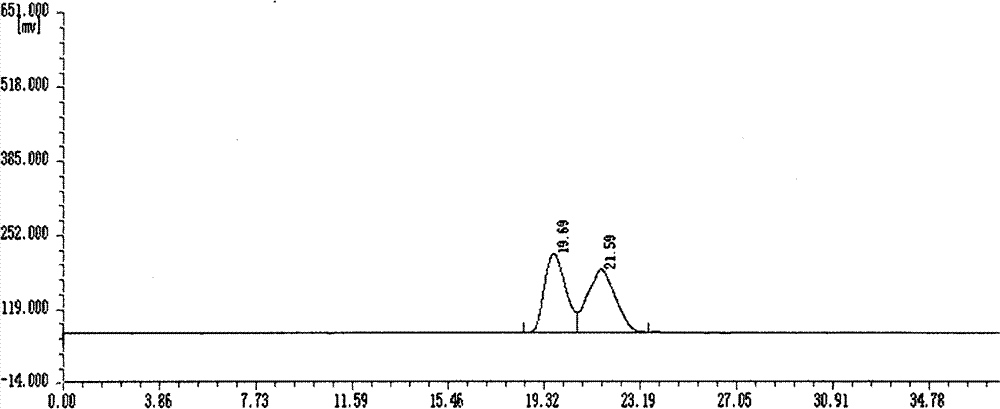
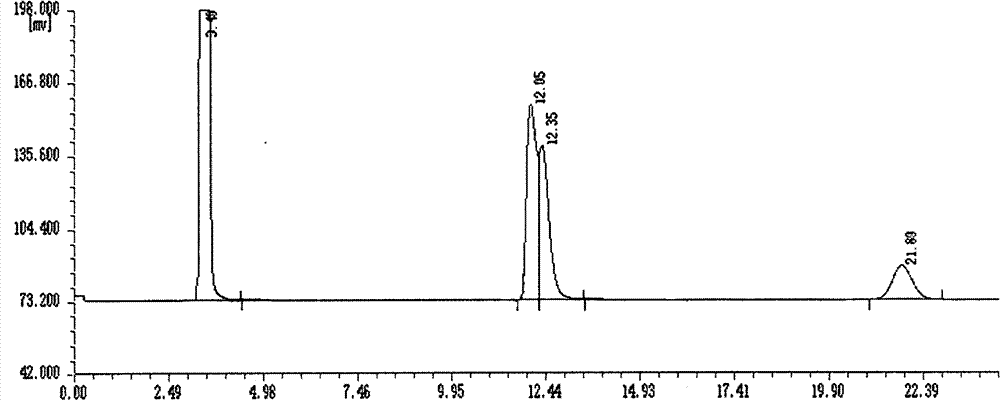
[0016] Add 1-(1-naphthyl)ethylamine and S-styroyl acetate in a molar ratio of 1:0.5 to a toluene solvent 10 times the volume of the chiral amine to form 1-(1-naphthyl)ethane Novozymes 435 was added at a mass fraction of 10% of the amine, and reacted for 48 hours at a reaction temperature of 30°C to obtain R-1-acetylnaphthylethylamine with a conversion rate of 50% and an e.e value >99%.
Embodiment 2
[0018] Add 1-(2-naphthyl)ethylamine and S-styroyl acetate in a molar ratio of 1:3 to a toluene solvent 10 times the volume of the chiral amine to form 1-(2-naphthyl)ethylamine Novozymes 435 was added at a mass fraction of 80% of the amine, and reacted for 12 hours at a reaction temperature of 70°C to obtain R-2-acetylnaphthylethylamine with a conversion rate of 50% and an e.e value >99%.
Embodiment 3
[0020] Add 1-(1-naphthyl)ethylamine and R-styroyl acetate in a molar ratio of 1:2 to a toluene solvent 10 times the volume of the chiral amine to form 1-(1-naphthyl)ethylamine Novozymes 435 was added at a mass fraction of 40% of the amine, and reacted for 24 hours at a reaction temperature of 70°C to obtain S-1-acetylnaphthylethylamine with a conversion rate of 50% and an e.e value >99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com