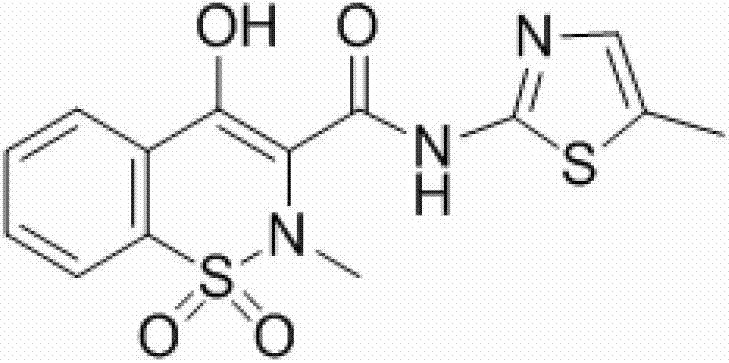
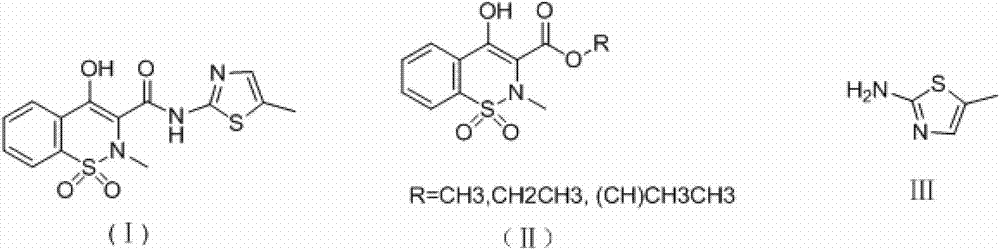
Synthesis method of meloxicam
A meloxicam and synthesis method technology, applied in the field of drug synthesis, can solve the problems of high toxicity of xylene, long reaction time, and large amount of solvent, and achieve the effects of less solvent amount, short reaction time, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 283g (1mol) 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-ethyl carboxylate-1,1-dioxide and 126g (1.1mol) 2-amino -5-Methylthiazole, then add 600mL dimethyl sulfoxide, stir, heat up to 189 degrees, distill off ethanol at the same time during the reaction, add 450mL ethanol to the reaction solution after 3 hours of reaction, cool to room temperature and filter to obtain Crude product, the crude product was recrystallized from tetrahydrofuran to obtain 323g of meloxicam, with a yield of 92% and a content of 99.4%.
Embodiment 2
[0022] Add 283g (1mol) 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-ethyl carboxylate-1,1-dioxide and 137g (1.2mol) 2-amino -5-Methylthiazole, then add 1000mL dimethyl sulfoxide, stir, heat up to 189 degrees, distill off ethanol at the same time during the reaction, react for 3 hours, add 1.6L ethanol to the reaction solution after the reaction is completed, cool to After filtering at room temperature, the crude product was obtained. The crude product was recrystallized from tetrahydrofuran to obtain 316 g of meloxicam, with a yield of 90% and a content of 99.6%.
Embodiment 3
[0024] Add 283g (1mol) 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-ethyl carboxylate-1,1-dioxide and 148g (1.3mol) 2-amino -5-Methylthiazole, then add 1000mL dimethyl sulfoxide, stir, heat up to 140°C, distill off ethanol at the same time during the reaction, add 1L ethanol to the reaction solution after 12 hours of reaction, cool to room temperature and filter to obtain Crude product, the crude product was recrystallized from tetrahydrofuran to obtain 306g of meloxicam, with a yield of 87% and a content of 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com