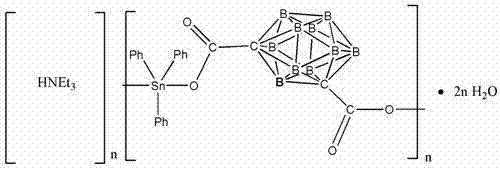
1, 7-meta dicarbadecaborane carboxylic acid triphenyl stannic chloride compound as well as preparation method and application of compound
A technology of meta-decaborane carboxylic acid and triphenyltin chloride, applied in tin organic compounds, organic chemistry, drug combination, etc., to achieve good fat solubility, low cost, and high anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Will 1.0mmol m-c 4 B 10 H 12 O 4 , 2.0mmol net 3 Soluble at 15ml CH 3 In OH, then add 2.0 mmol pH 3 Sncl's CH 3 In the OH solution, after the normal temperature is 0.5h, the 1.0 mmol3,5-pyridine di carboxylic acid is added to the above-mentioned hybrid solution, and the normal temperature will be stirred for 12 hours.Filter, the filter fluid is static at room temperature.After the three-year period, the colorless crystal is obtained, and the melting point is 226-228 ° C.The production rate of this compound was 69.8%.
[0016] The organic tin compounds of the present invention are analyzed by infrared spectrum and X-single crystal diffraction analysis. The results are as follows: IR (KBR, CM -1 ): V = 1643.1, 1384.4 (COO), 543.9 (SN-C), 454.9 (SN-O).
[0017] Crystal data: The crystal system of this chemical compound belongs to the orthogonal crystal system, the space group is PBCA, and the crystal parameters are: A = 20.2440 (18)?, B = 18.2560 (15)?, C = 20.2435 (19)?, Α =9...
Embodiment 2
[0019] Will 1.0mmol m-c 4 B 10 H 12 O 4 (1,7-interval binocarboline carboxylic acid), 2.0 mmol net 3 Soluble at 15ml CH 3 In OH, then add 2.0 mmol pH 3 Sncl's CH 3 In the OH solution, after the normal temperature is 0.5h, the 1.0 mmol3,5-pyridine di carboxylic acid is added to the above-mentioned hybrid solution, and the normal temperature will be mixed for 16h.Filter, the filter fluid is static at room temperature.After the three-year period, the colorless crystal is obtained, and the melting point is 226-228 ° C.The production rate of this compound was 65.3%.
Embodiment 3
[0021] Will 1.0mmol m-c 4 B 10 H 12 O 4 , 2.0mmol net 3 Soluble at 15ml CH 3 In OH, then add 2.0 mmol pH 3 Sncl's CH 3 In the OH solution, after the normal temperature is 0.5h, the 1.0 mmol3,5-pyriarine di carboxylic acid is added to the above-mentioned hybrid solution, and the normal temperature will be stirred for 20 hours.Filter, the filter fluid is static at room temperature.After the three-year period, the colorless crystal is obtained, and the melting point is 226-228 ° C.The production rate of this compound was 64.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com