Secretory protein with migration activity and application thereof
A protein and homology technology, applied in the field of genetic engineering, can solve the problems of large investment and heavy workload, and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
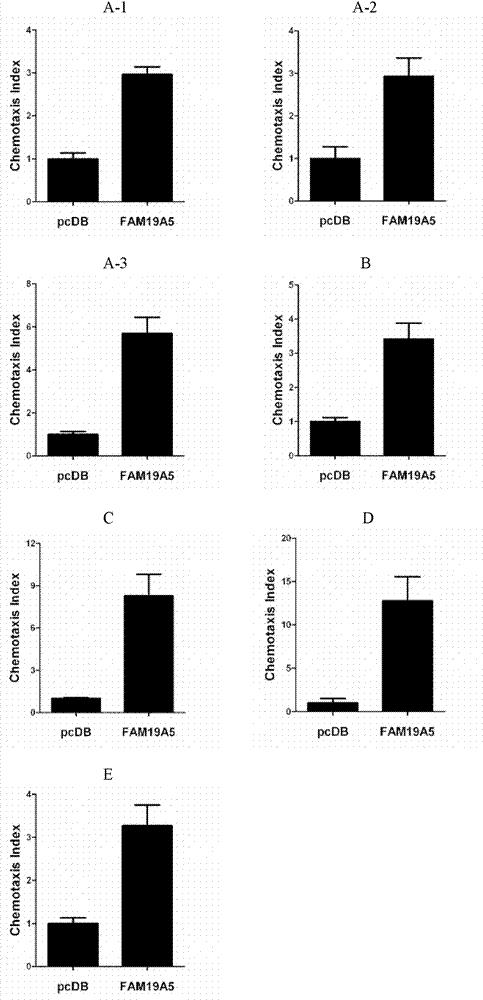
Examples
Embodiment 1
[0070] Embodiment 1, construction pcDNA3.1-FAM19A5-myc-his6 fusion protein expression plasmid
[0071] The pcDNA3.1-FAM19A5-myc-his6 plasmid was constructed to express the FAM19A5-myc-his6 fusion protein.
[0072] 1. Method:
[0073] The FAM19A5 coding sequence (SEQ ID NO: 2; 3; 4) was respectively inserted into the pcDNA3.1-myc-his6 (Invitrogen Company) expression vector to construct the pcDNA3.1-FAM19A5-myc-his6 expression plasmid. After the correctness of the plasmid was verified by sequencing, the plasmid was amplified, and the plasmid was extracted with the Axygen plasmid extraction kit for cell transfection.
[0074] 2. Results:
[0075] The sequence of the coding region was correct by DNA sequencing.
Embodiment 2
[0076] Example 2, plasmid transfection cells to obtain FAM19A5 expression supernatant
[0077] 1. Method:
[0078] HEK 293T cells adjusted to 6×10 5 Cells / 2ml concentration in 6-well plate at 37°C 5% CO 2 Culture for 24 hours for transfection, pcDNA3.1-FAM19A5 (BC039396.1; or NM_001082967.1; or NM_015381.5)-myc-his6DNA 2μg, vigofect (Vigofect Biotechnology Co., Ltd.) 2μl, the mixture was dripped slowly Into the prepared cells, at the same time set pcDNA3.1-myc-his6 empty vector transfection cells as a control, change the serum-free medium HEKG after 6 hours, culture at 37°C for 48 hours, and harvest the supernatant after transfection.
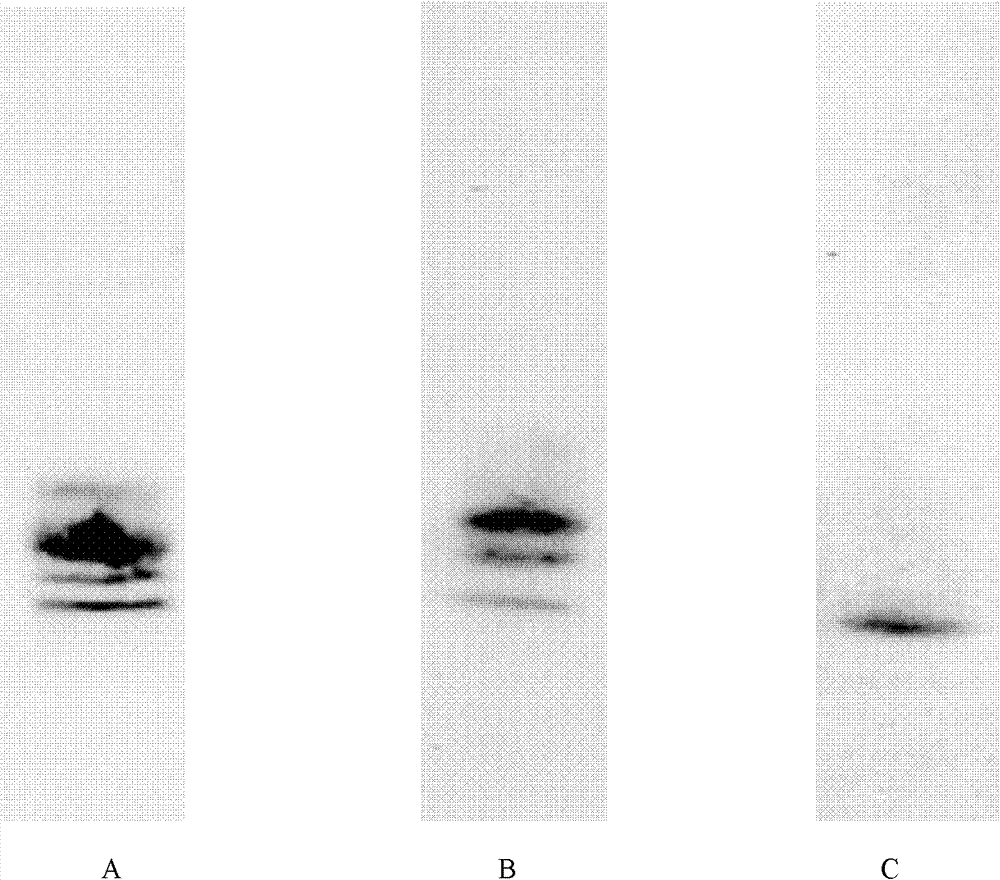
[0079] Use Western Blotting to check the expression of the target protein:
[0080] After 12.5% SDS-PAGE electrophoresis, the protein was transferred to the nitrocellulose membrane with Tris-glycine electrotransfer solution, after adding Anti-c-myc antibody (MBL company) to act, then adding IRDyeTM 800-labeled anti-mouse IgG (LICOR Bioscie...
Embodiment 3
[0084] Example 3, the separation of human peripheral blood mononuclear cells
[0085] 1. Method:
[0086] Concentrated white blood cells were provided by Beijing Blood Center, and human peripheral blood mononuclear cells were obtained using lymphocyte separation medium (Shanghai Huajing Bio-Technology Co., Ltd.). Culture with RPMI 1640 (Life Technologies, Inc.) containing 10% heat-inactivated fetal bovine serum, 100 U / ml penicillin, and 100 μg / ml streptomycin.
[0087] 2. Results:
[0088] Human peripheral blood mononuclear cells were obtained and cultured in vitro for future use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com