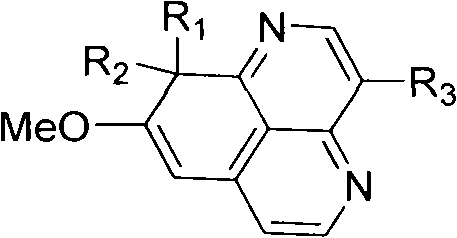
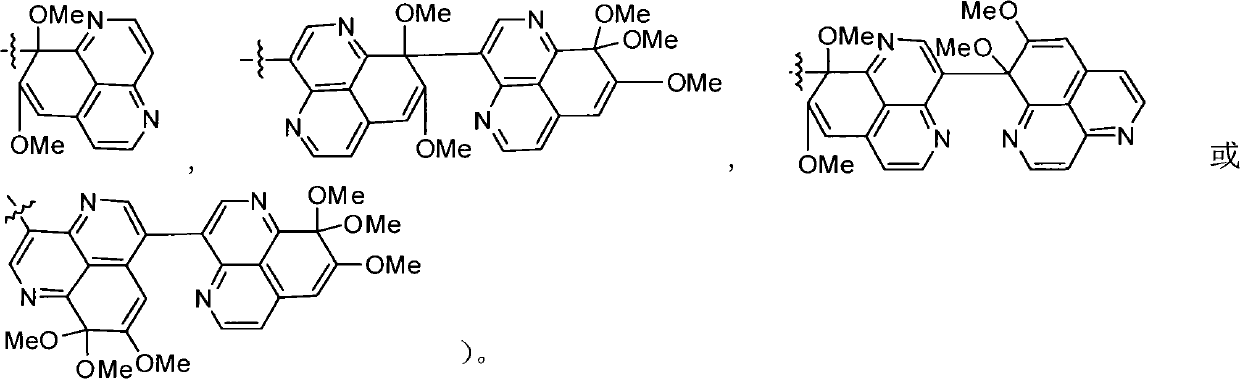
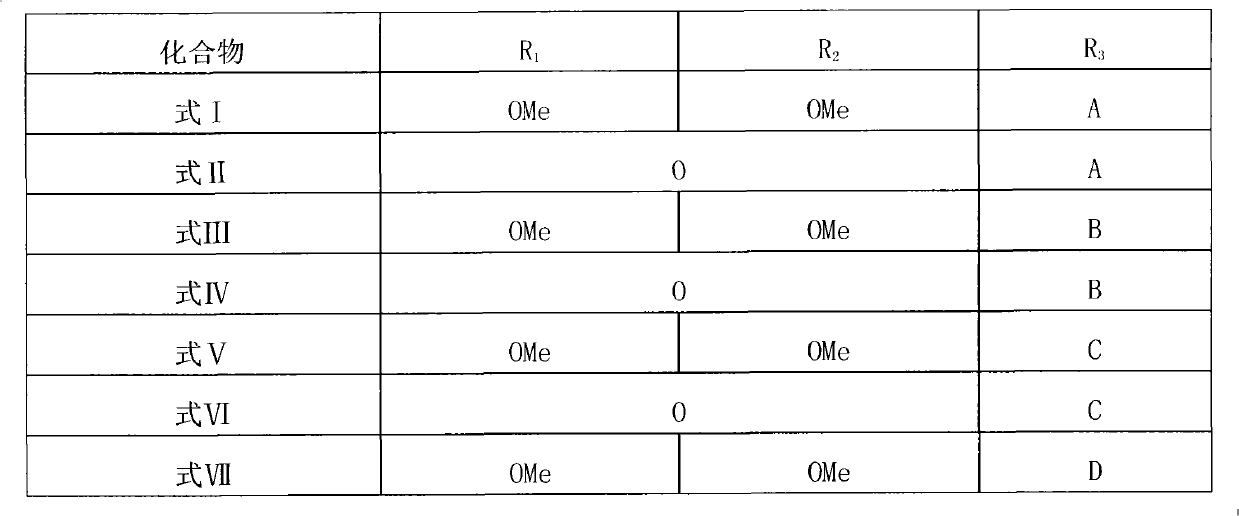
Polymerization aaptamine alkaloid compounds, preparation method thereof and application of polymerization aaptamine alkaloid compounds
A technology of compounds and alkaloids, applied in the field of medicine, can solve problems such as no anti-tumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the separation and extraction of compound I-VII
[0044] 1. Obtaining the extract
[0045] After crushing the sponge sample (wet weight 2.0kg) frozen and stored, it was extracted by dynamic cold immersion with methanol three times at room temperature, soaked for 3 days each time; Total extract 80g.
[0046] 2. Separation and purification of compounds
[0047]After the extract (80.0 g) was dissolved with methanol solvent, add 80 g of 100-200 mesh silica gel (Qingdao Ocean Chemical Group Co., Ltd. product) to mix the sample, remove the solvent under reduced pressure, and use silica gel column chromatography to analyze the mixture with 100% sherwood oil, petroleum Ether-acetone (20:1, 15:1, 10:1, 5:1, 3:1, 2:1, 1:1), 100% acetone, 100% methanol as solvent for gradient elution, divided into 8 share. Fr-6 (2.5 g, petroleum ether-acetone 1:1 eluate) was subjected to forward silica gel column chromatography, with petroleum ether / acetone (6:1, 3:1, 2:1, 1:1) g...
Embodiment 2
[0098] Embodiment 2: the test of antitumor activity in vitro
[0099] Cell Proliferation Inhibitory Activity Test
[0100] 1. Experimental samples and experimental methods
[0101] Preparation of the test sample solution: the test sample is the pure compound I-VII isolated and refined in Example 1 above. Accurately weigh an appropriate amount of sample, and prepare a solution with the required concentration with methanol for activity measurement.
[0102] Cell lines and subculture of cells: Human liver cancer BEL-7402 cells, human cervical cancer HeLa cells, chronic myelogenous leukemia K562 cells, mouse leukemia P388 cells and other cancer cell lines are used. All kinds of cells were subcultured in RPMI-1640 medium containing 10% FBS at 37°C in an incubator filled with 5% carbon dioxide.
[0103] Cell Proliferation Inhibitory Activity Test Method
[0104] Lissamine rhodamine B (SRB) method: Take human cervical cancer HeLa cells and human liver cancer BEL-7402 cells in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com