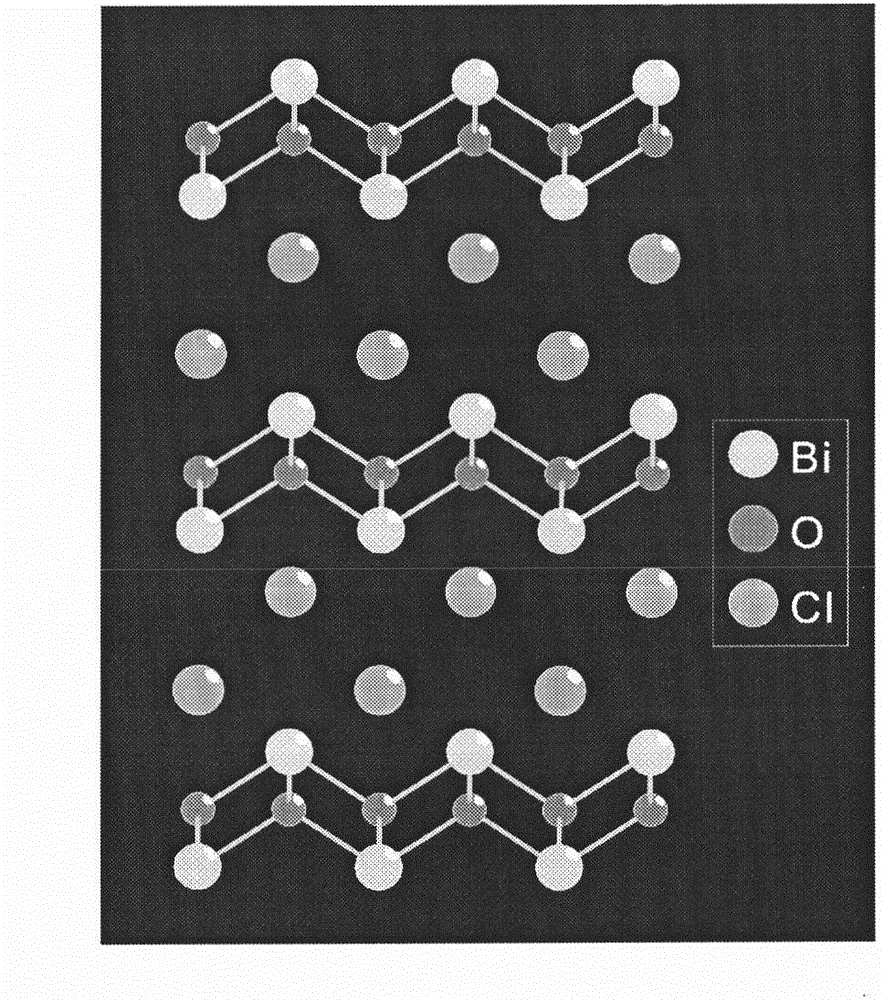
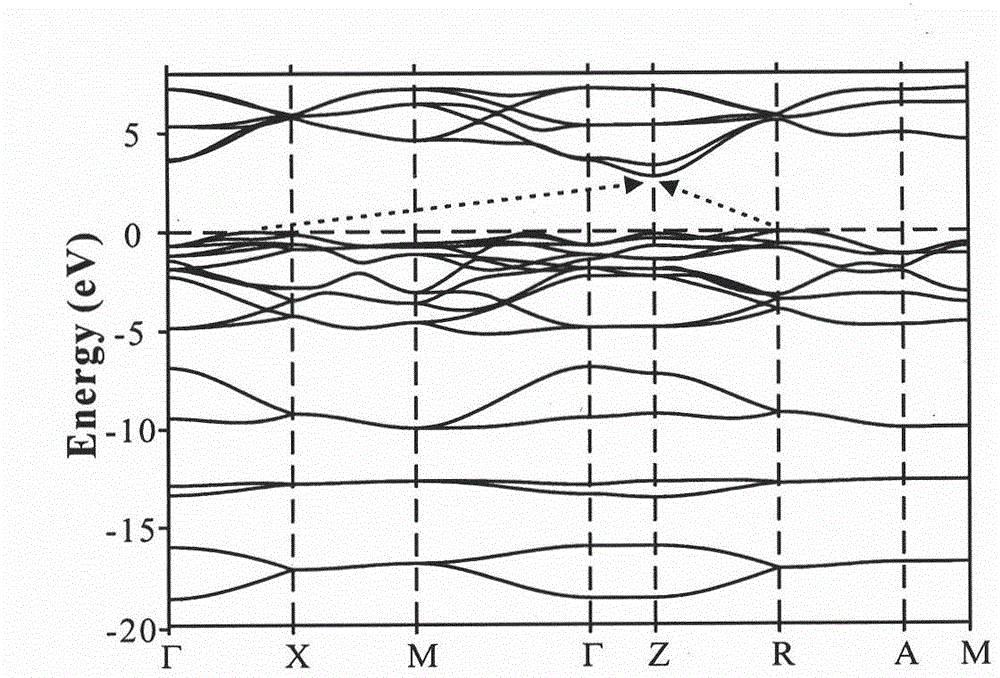
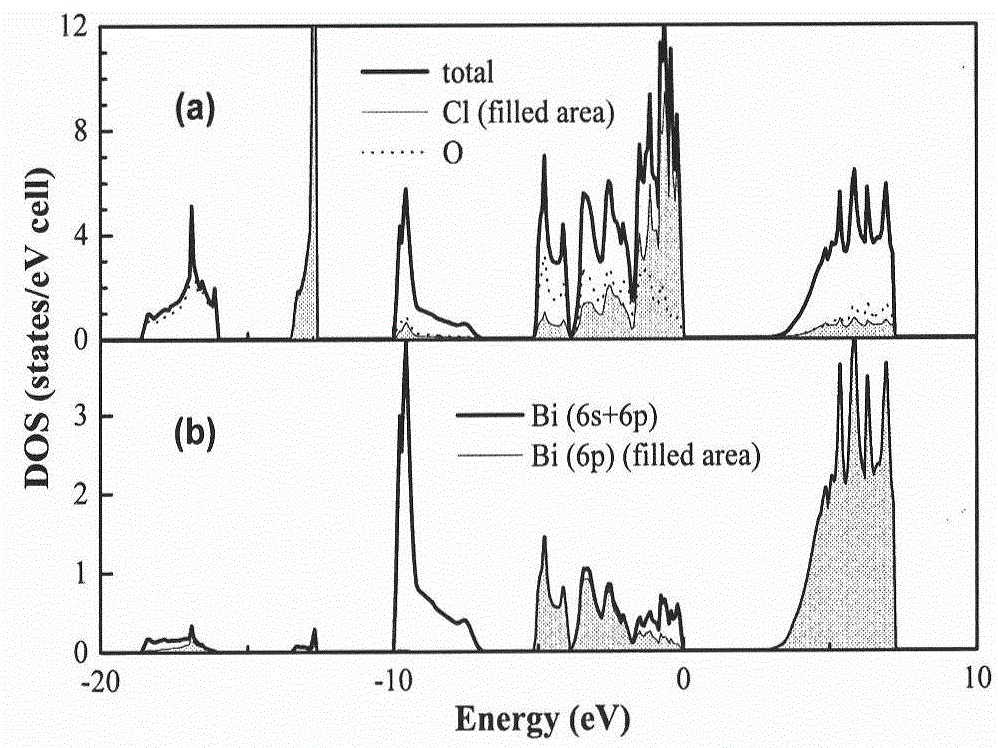
Oxyhalide photo-catalytic material and preparation method thereof
A photocatalytic material, oxyhalide technology, applied in chemical instruments and methods, physical/chemical process catalysts, separation methods, etc., can solve the problem of low quantum yield, unsatisfactory photocatalytic effect, and poor powder photocatalytic activity And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Bi 2 o 3 The powder is dissolved in the corresponding concentrated halogen acid, adjusted to pH = 3 with dilute ammonia water, filtered for many times until no X-ions are detected (tested with silver nitrate solution), and the precipitate is dried at 80°C to obtain the finished nanolight Catalytic powder.
[0042] The specific process of the BiOX (X=Cl, Br, I) photocatalytic material of load can be: get 2g BiOX (X=Cl, Br, I) catalyst powder and insert in the beaker that contains 100ml deionized water, add the methanol of 10ml Hole sacrificial agent and appropriate amount of AgNO 3 or H 2 PtCl 6 ·6H 2 O, under a 300W mercury lamp for 85-10 hours while vigorously stirring, after several times of filtration, washing, and drying, the photocatalytic material loaded with nano-Ag or Pt particles can be obtained.
[0043] The experiment of photocatalytic degradation of organic substance methyl orange shows that the UV photocatalytic performance of BiOX (X=Cl, Br) is bette...
Embodiment 2
[0048] Bi of the stoichiometric ratio 2 o 3 and BiX 3 (X=Cl, Br, I) was placed in a quartz tube, vacuumized and packaged, and heated at 550° C. for 12 hours. After cooling, open the tube and grind to get the finished photocatalytic powder.
[0049] Photocatalytic degradation of dye methyl orange, photolysis of water to produce hydrogen, degradation of harmful substance formaldehyde and bactericidal test results are about 40% of those in Embodiment 1, which may be due to the smaller specific surface area of the sample prepared by the solid phase method.
Embodiment 3
[0051] Take 100ml of deionized water in a beaker, adjust the pH of the solution to less than 1 with the corresponding halogen acid, then heat the solution to about 70°C, while stirring continuously, slowly add 1mol of bismuth nitrate-halogen acid solution, and at the same time slowly add 15% NaOH solution to neutralize the HNO produced by hydrolysis 3 , maintain the pH value at around 2, and the whole process lasts for about 1 hour. During this period, the temperature was kept constant at about 75°C, continued to stir for 10 minutes, cooled, filtered, washed, and dried at 80°C to obtain the finished photocatalytic powder.
[0052] The performance test result is basically the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com