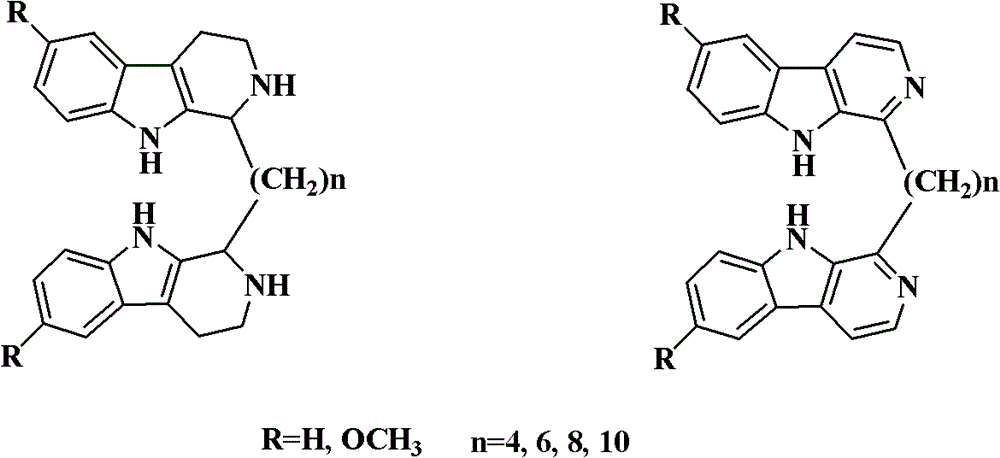
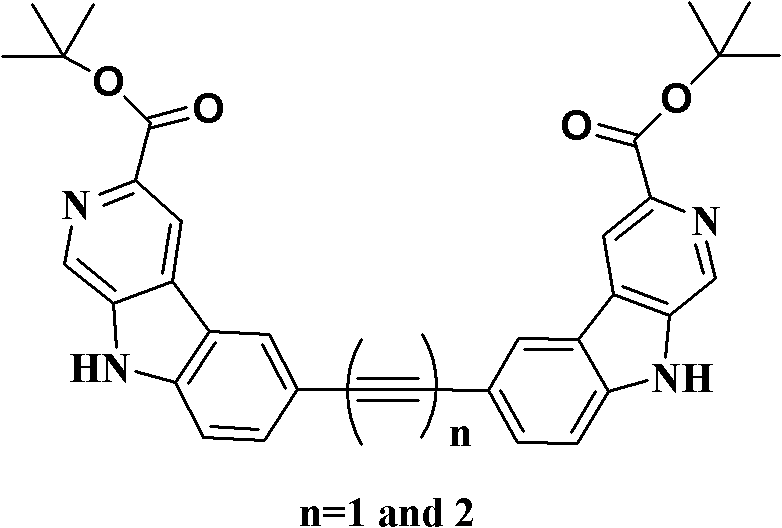
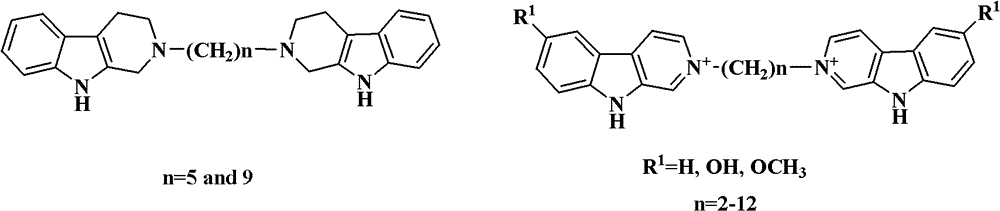
Di-beta-carboline alkali compound and preparation method, medicinal composition and application thereof
A technology of compound and carboline base, which is applied in the field of application of bis-β-carboline base compound and its pharmaceutically acceptable salt, in the preparation of anti-tumor drugs, and can solve the problems of undisclosed effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0128]
[0129] Ethyl 1-(pyridin-3-yl)-β-carboline-3-carboxylate:
[0130] (a) Synthesis of ethyl 1-(pyridin-3-yl)-1,2,3,4-tetrahydro β-carboline-3-carboxylate
[0131] Add L-tryptophan ethyl ester (8.04 g, 30 mmol) and isopropanol (84 mL) into a 250 ml round bottom flask, and stir at room temperature until the solution is clear. Subsequently, 3-pyridinecarbaldehyde (36 mmol) was added, and the mixture was heated to reflux for reaction, followed by TLC detection. After the reaction was completed, it was concentrated to dryness under reduced pressure to obtain a light yellow solid. Dissolve the above solid in water, NaHCO 3 Alkalinization, extraction with ethyl acetate, combined organic phases, washing with water, washing with saturated brine, drying over anhydrous sodium sulfate, and concentrating to dryness under reduced pressure to obtain an oily product, which can be directly used in the next step without purification.
[0132] (b) Synthesis of ethyl 1-(pyridin-3-yl)-...
preparation example 2
[0135]
[0136] 1-(pyridin-3-yl)-β-carboline:
[0137] L-tryptophan (10mmol) and glacial acetic acid (20ml) were mixed, heated until completely dissolved, then 3-pyridinecarbaldehyde (10.6mmol) was added, the reaction mixture was heated to reflux, followed by TLC detection. After the reaction is completed, the reaction mixture is poured into boiling water, then potassium dichromate (20mmol) is added, heating is continued for 20 minutes, the heating is stopped, the reaction solution is cooled to room temperature, and then anhydrous Na 2 SO 3 (2.7g) with constant stirring, then adjust the pH value to about 10 with solid NaOH, extract with ethyl acetate, combine the organic phases, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to dryness under reduced pressure to obtain yellow Solid, recrystallized from ethyl acetate to obtain 1.97 g of light yellow solid, yield 80%. 1 H NMR (300MHz, CDCl 3 ): δ10.11 (1H,...
preparation example 3
[0139]
[0140] 1-(thiophen-3-yl)-β-carboline:
[0141] Mix L-tryptophan (10 mmol) and dichloromethane (100 ml), add phenylacetaldehyde (1.05 ml) and trifluoroacetic acid (1.5 ml) under stirring at room temperature, continue stirring at room temperature, and track and detect by TLC. After the reaction was completed, the reaction mixture was concentrated under reduced pressure to about 30 ml, filtered, and carefully washed with ether to obtain a solid. The solid was subsequently dissolved with glacial acetic acid, and KMnO 4 (30mmol) reflux, TLC tracking detection. After the reaction was completed, the solvent was evaporated under reduced pressure, the residue was poured into water, alkalized with sodium bicarbonate, extracted with ethyl acetate, the organic phases were combined, washed with water, washed with saturated brine, anhydrous Na 2 SO 4 Dry, decolorize with activated carbon, filter, and concentrate the filtrate to dryness under reduced pressure, and perform sili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com