Preparation method of compound amino acid (15) dipeptide (2) injecta
A compound amino acid and injection technology is applied in the field of preparation of compound amino acid dipeptide injection, which can solve the problems of low quality, reduced medication safety, and inability to overcome product stability problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 compound amino acid (15) dipeptide (2) injection:
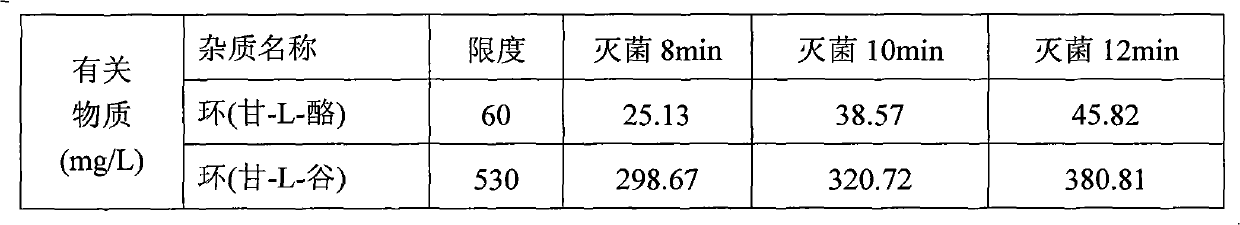
[0032] Weigh each amino acid raw material according to the ratio in the table below, 1000ml contains:
[0033]
[0034]Under the protection of nitrogen, take 3000 ml of water for injection with a total volume of 60%, heat it to 60°C, add the prescribed amount of glycyl-L-glutamine and glycyl-L-tyrosine in sequence, dissolve the Finally, add the prescribed amount of arginine, stir and dissolve, quickly add the prescribed amount of aspartic acid, glutamic acid, leucine, isoleucine and phenylalanine that have passed through a 80 mesh sieve, and stir to dissolve. , finally add the prescribed amount of alanine, histidine, lysine acetate, methionine, proline, serine, threonine, tryptophan and valine, stir to dissolve, and use citric acid Adjust the pH to 5.4-5.8, add the full amount of 0.10% (w / v) activated carbon, stir at 60°C for 30 minutes, decarburize and filter, dilute the volume with...
Embodiment 2
[0036] The preparation of embodiment 2 compound amino acid (15) dipeptide (2) injection:
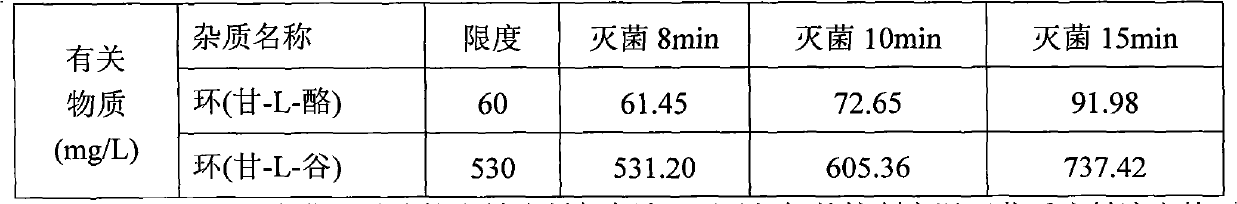
[0037] According to the feeding ratio of Example 1, under the protection of nitrogen, take 3000 milliliters of water for injection with a total volume of 60%, heat it to 60 ° C, add the prescribed amount of arginine, add glycyl-L-glutamine after dissolving Amide and Glycyl-L-Tyrosine, after stirring and dissolving, quickly add aspartic acid, glutamic acid, leucine, isoleucine and phenylalanine passing through a 80-mesh sieve, Stir to dissolve the clear, finally add the prescribed amount of alanine, histidine, lysine acetate, methionine, proline, serine, threonine, tryptophan and valine, stir to dissolve the clear, use Citric acid was used to adjust the pH to 5.4-5.8, and the total amount of 0.10% (w / v) activated carbon was added, stirred at 60°C for 30 minutes, decarbonized and filtered, and the volume was adjusted to 5000 ml with water for injection, and passed through a 0.22 micron fil...
Embodiment 3
[0039] Preparation of embodiment 3 compound amino acid (15) dipeptide (2) injection:
[0040] According to the feeding ratio of Example 1, under the protection of nitrogen, take 3000 milliliters of water for injection with a total volume of 60%, heat it to 60 ° C, add the prescribed amount of glycyl-L-glutamine and glycyl-L- Tyrosine, after dissolving, add arginine, stir to dissolve and dissolve, then add the prescribed amount of alanine, histidine, lysine acetate, methionine, proline, serine, threonine . Use citric acid to adjust the pH to 5.4-5.8, add 0.10% (w / v) activated carbon in full amount, stir at 60°C for 30 minutes, decarbonize and filter, dilute to a total volume of 5000 ml with water for injection, and pass through a 0.22-micron filter membrane , filled with 500ml / bottle, filled with nitrogen, stoppered, and covered with aluminum. Sterilize the product at 121±1°C for 12 minutes according to the set program. After the sterilized product passed the light inspectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com