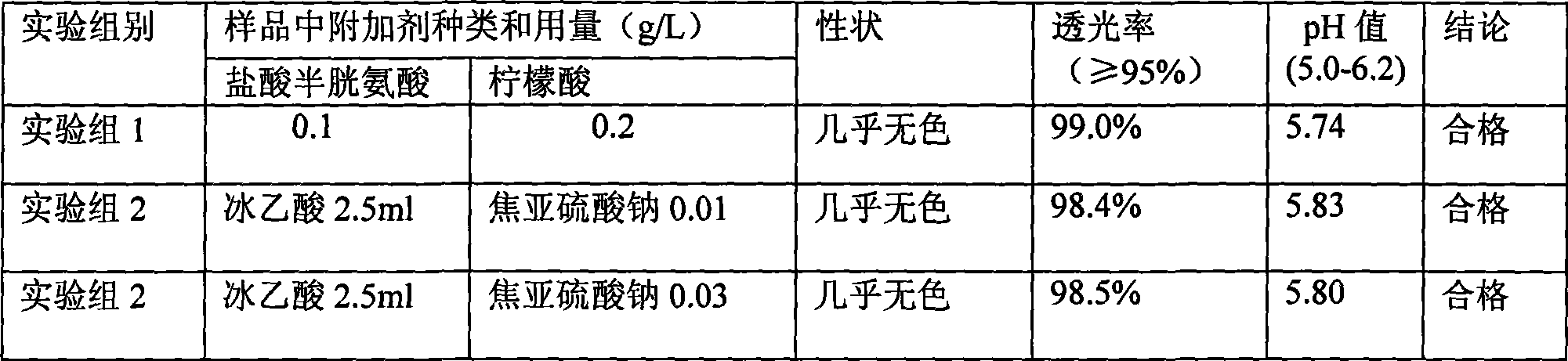
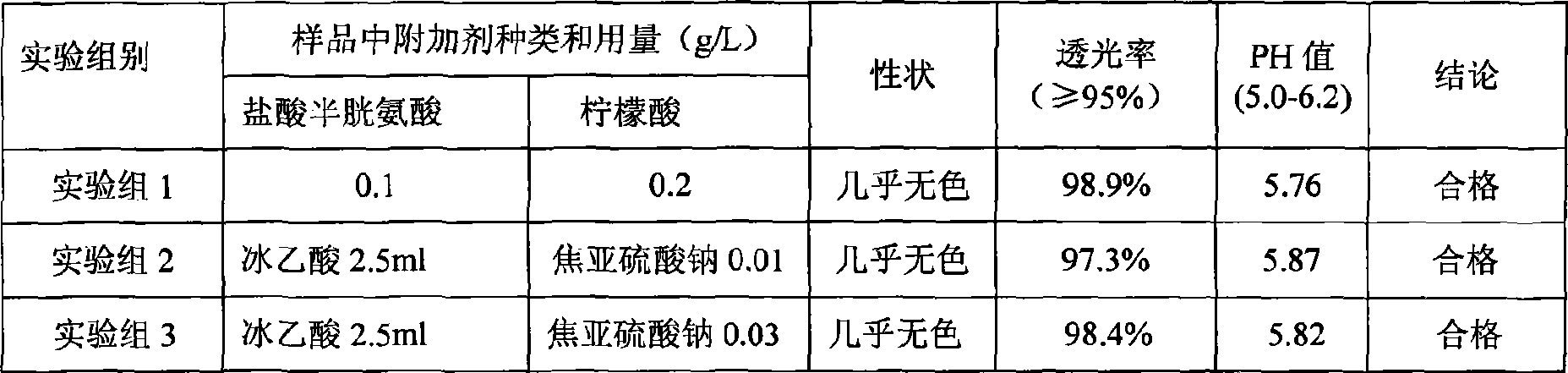
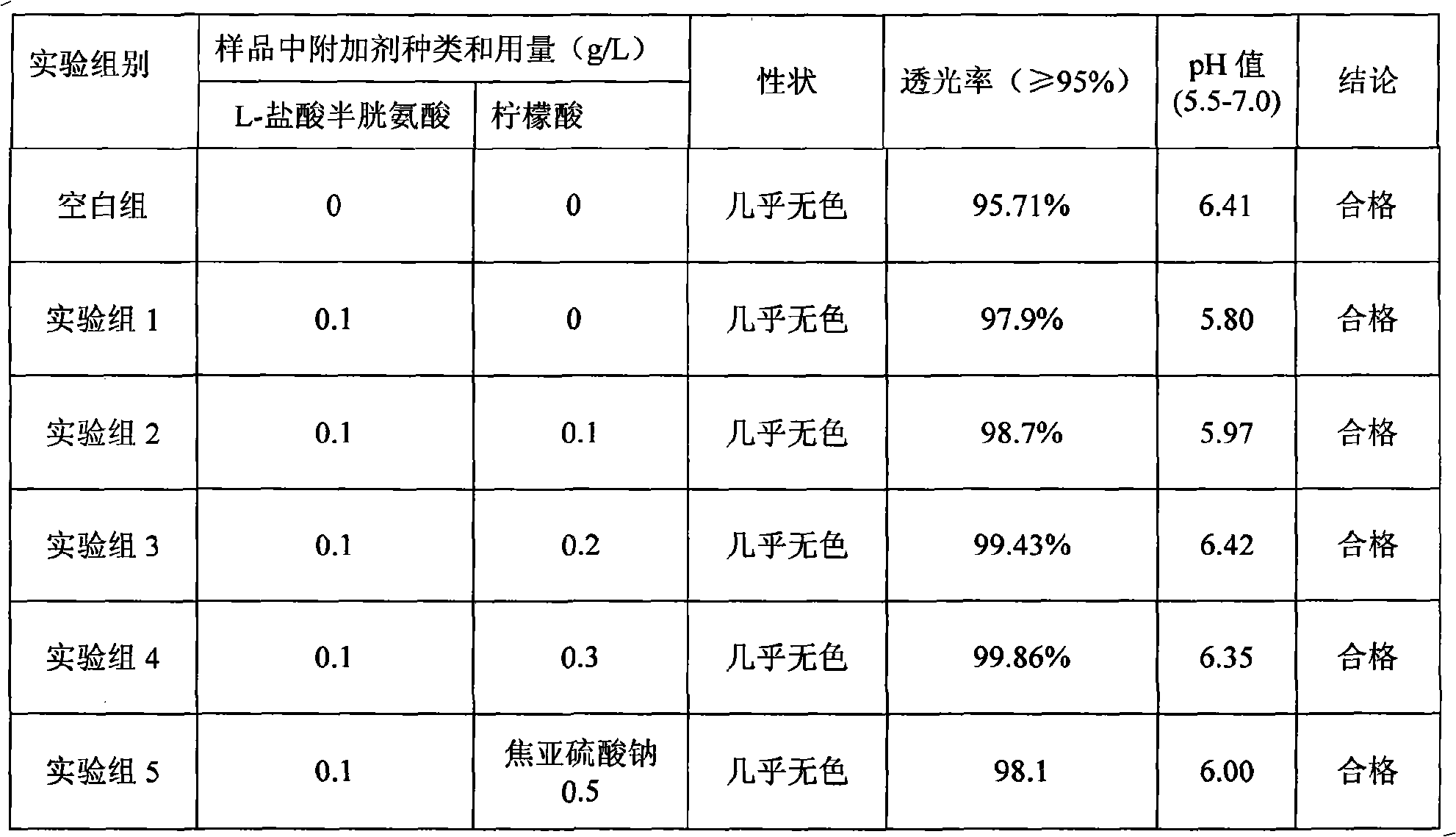
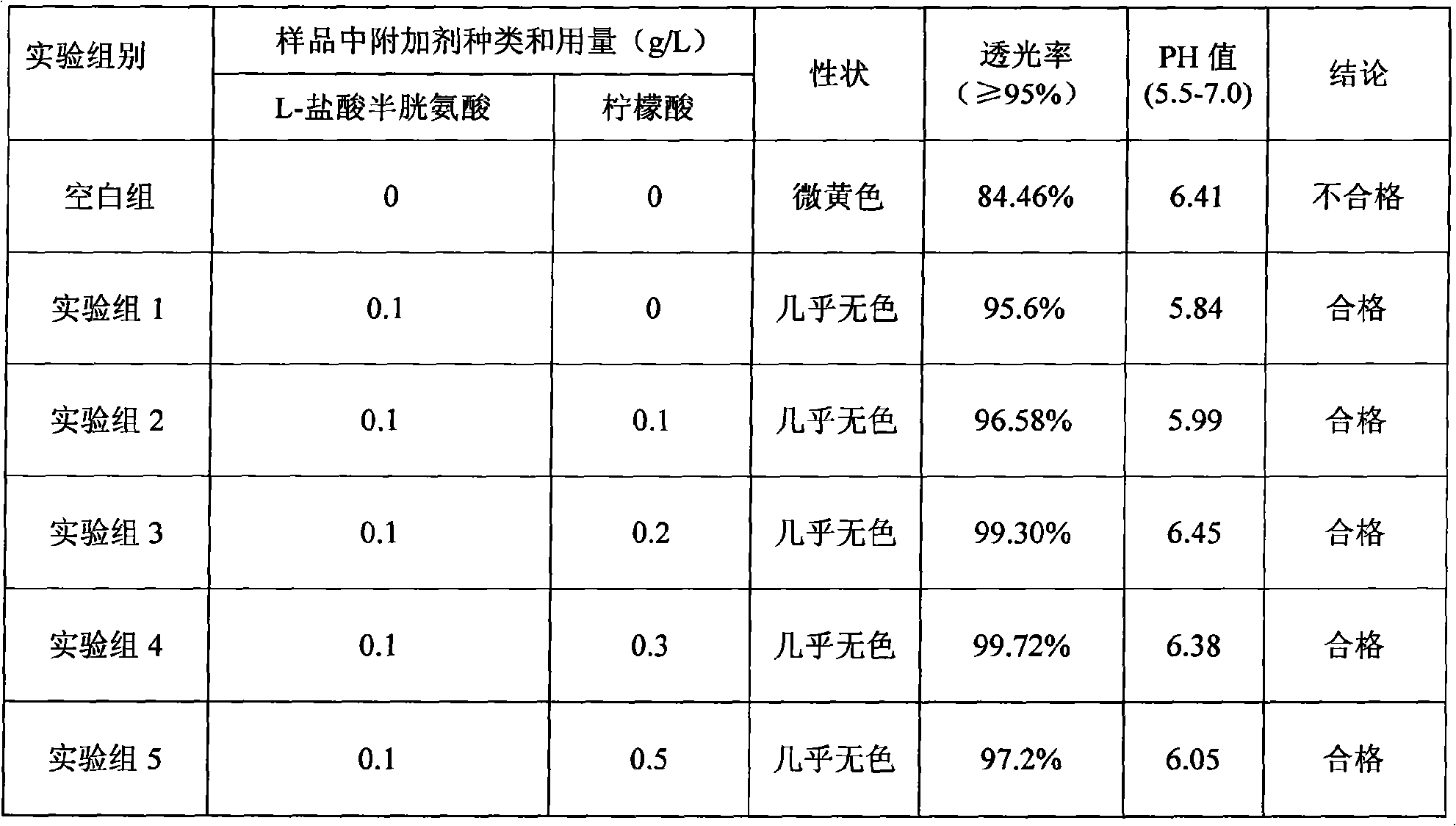
Patents
Literature
60 results about "LYSINE ACETATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An acetate salt of L-lysine. L-Lysine is an essential amino acid in human. It is metabolized to acetyl-CoA. It is widely used as an ingredient in infusion.
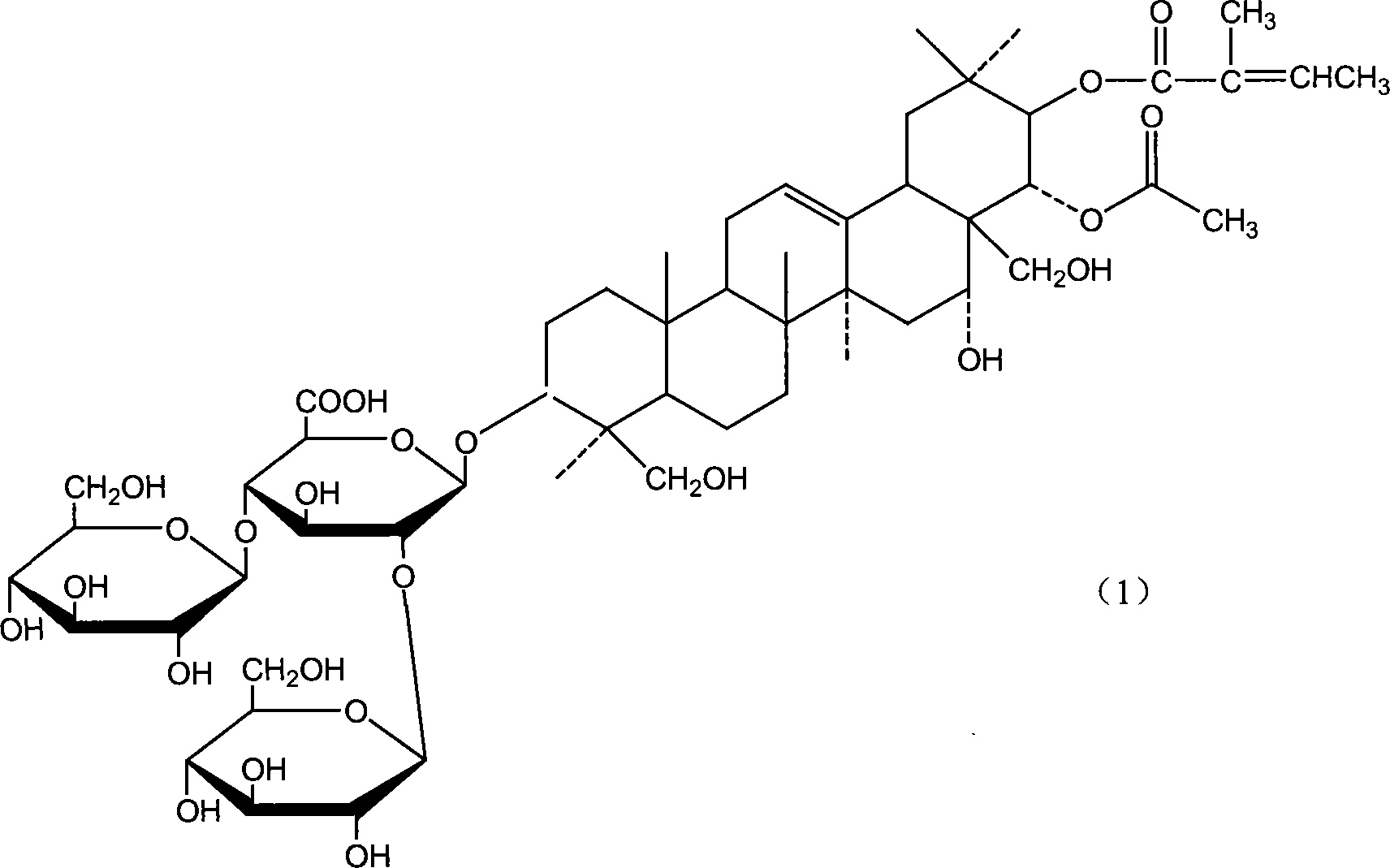
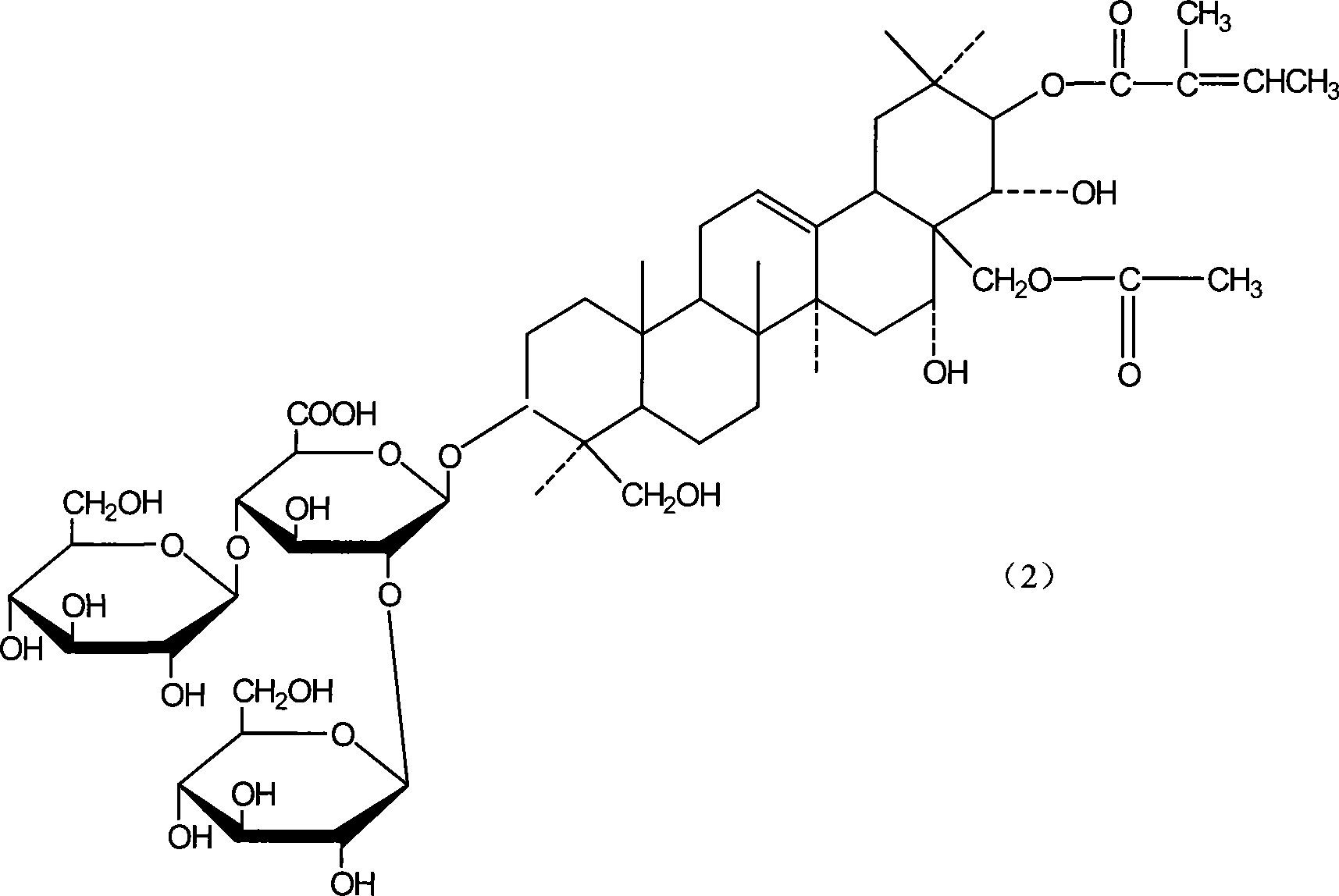
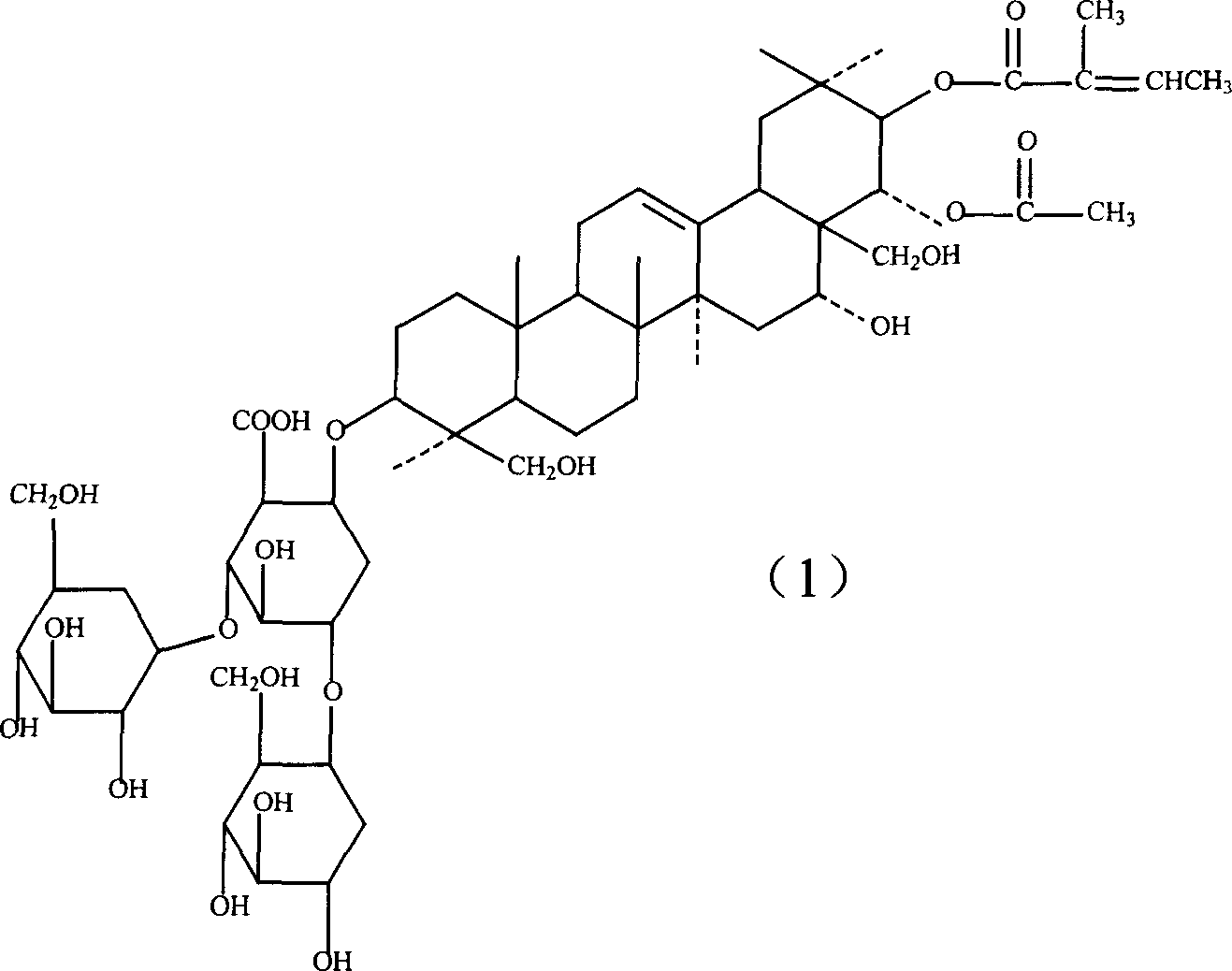
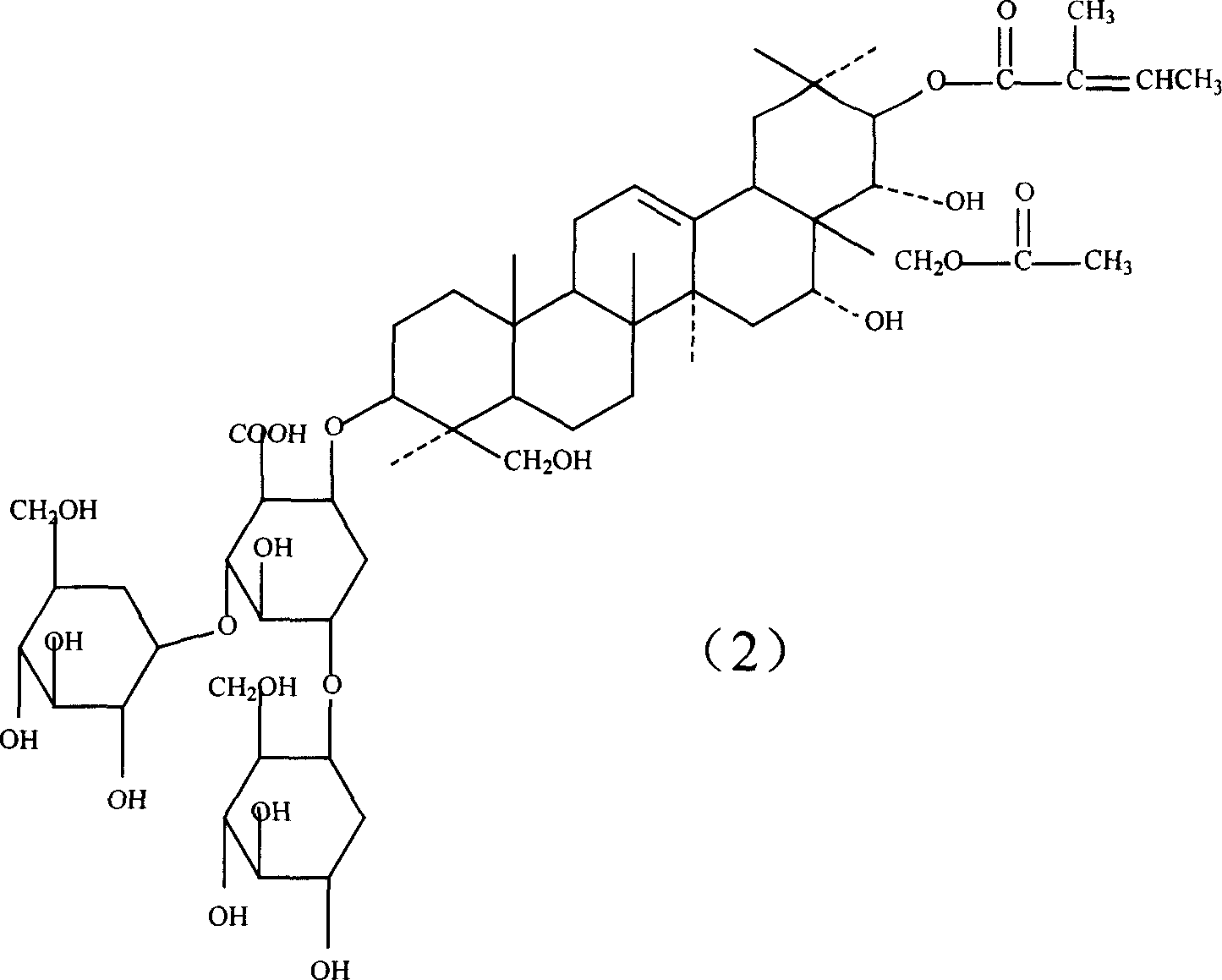
Notoginsenoside pharmaceutical composition and method for preparing the same and use thereof
ActiveCN101134042ALess irritatingEliminate side effectsOrganic active ingredientsAntipyreticIrritationDrug carrier
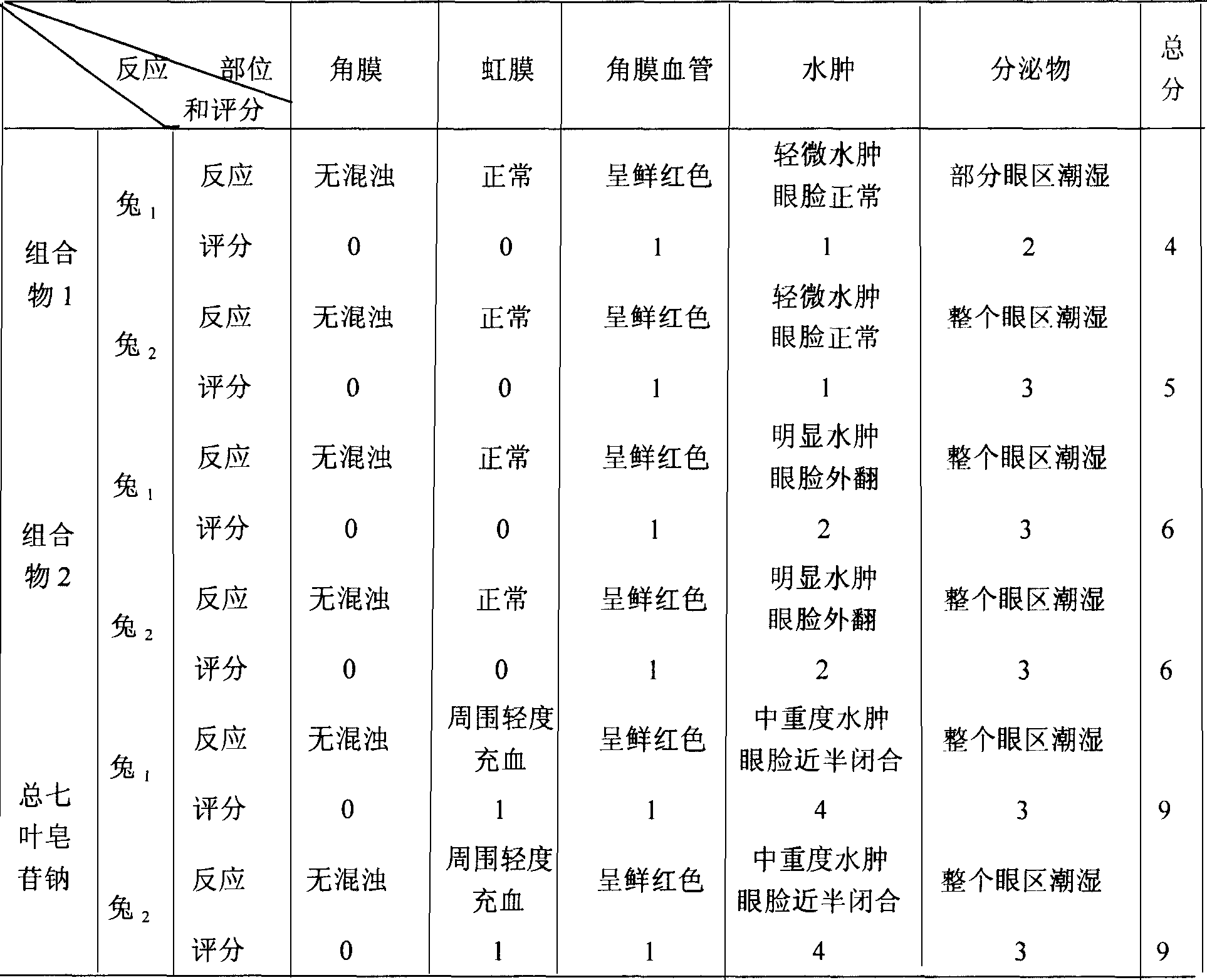
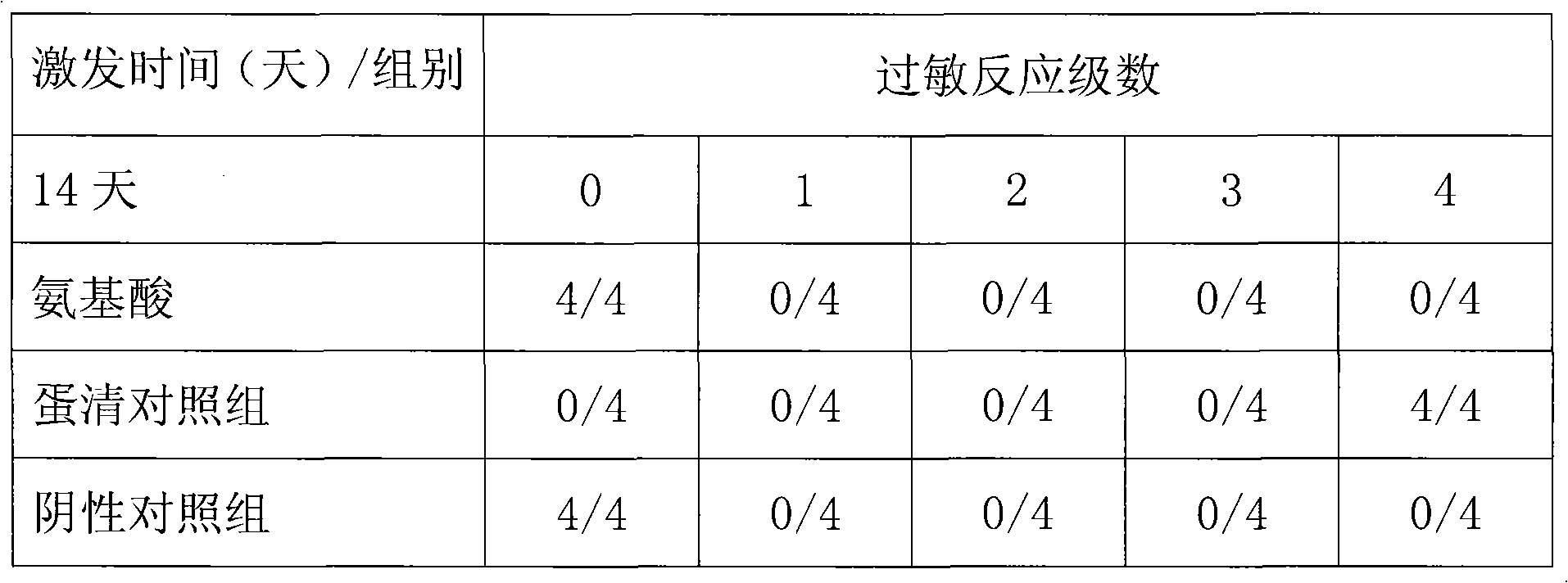
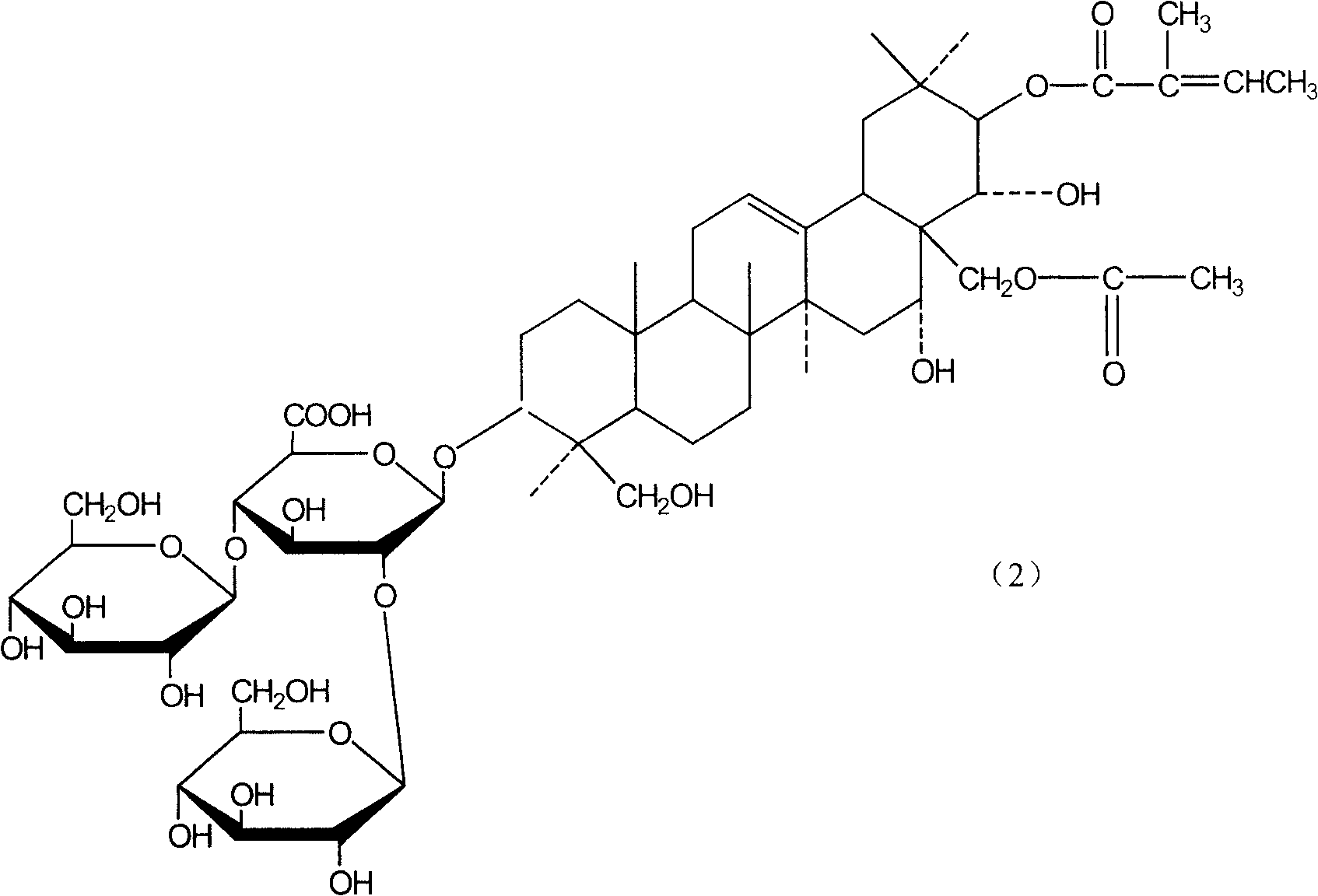
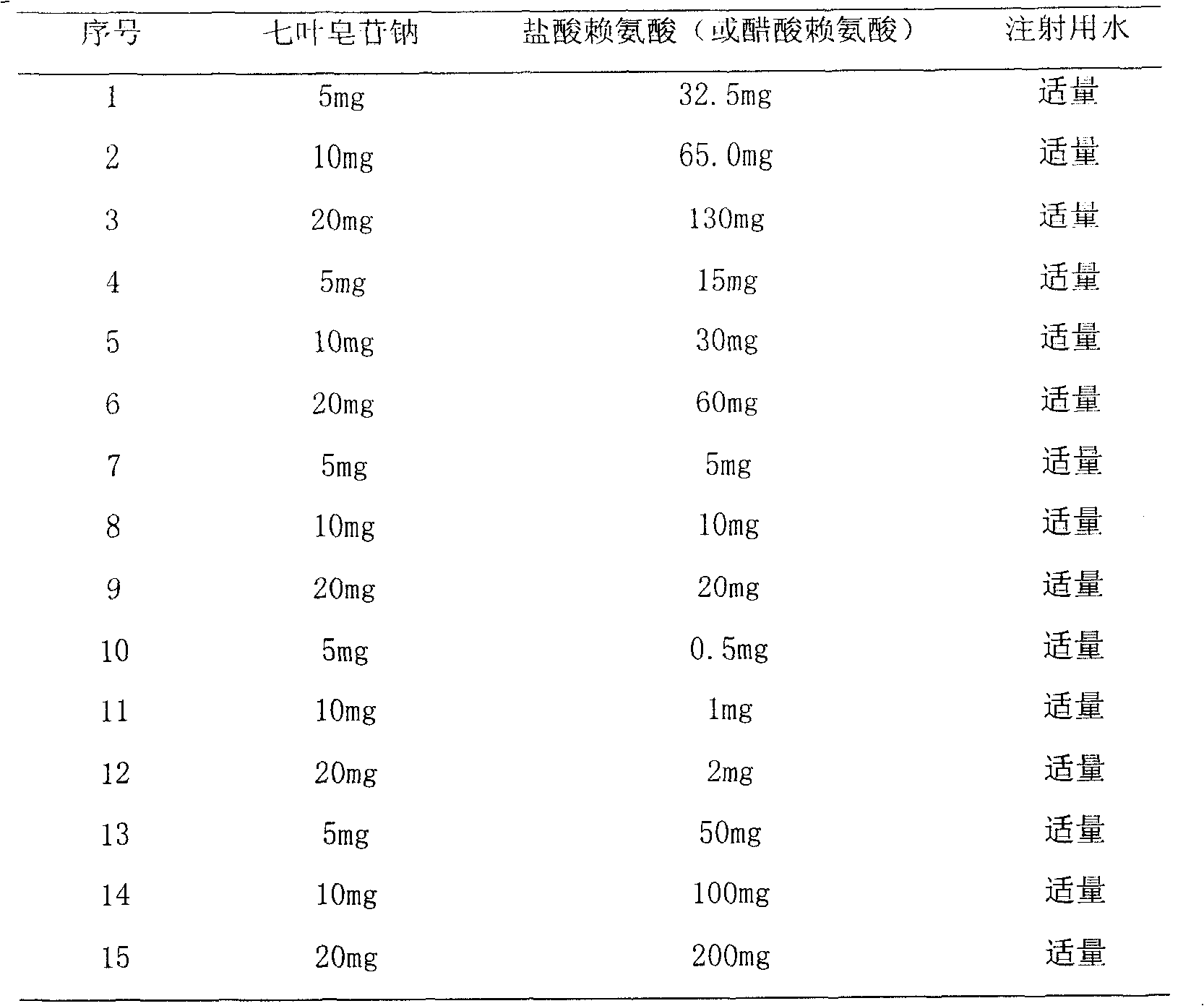
The present invention relates to medicine composition of aescin and its preparation process and use. The aescin with purity over 90 % may be compounded with medicine carrier to form medicine composition with high synergistic effect, obviously reduced irritation on blood vessel and muscle, obviously raised medicinal effects of resisting inflammation, resisting exudation, improving blood circulation, etc. The medicine composition has low irritation, high safety, high therapeutic index and other features.
Owner:WUHAN AIMIN PHARMA
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439031ASolve the problem of trace oxygenSolve the oxygen redissolution problemOrganic active ingredientsMetabolism disorderAntioxidantArginine
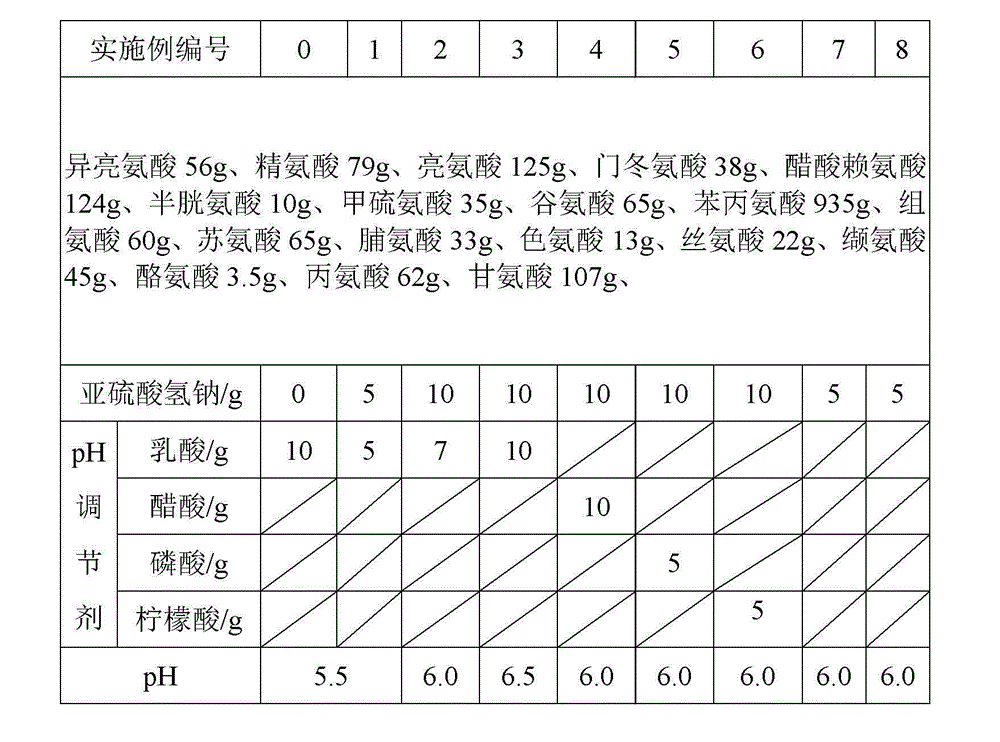
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-II) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 1.50 of aspartic acid, 2.50 of glutamic acid, 1.90 of serine, 3.00 of histidine, 3.50 of glycine, 2.50 of threonine, 7.20 of alanine, 4.90 of arginine, 0.20 of tyrosine, 0.20 of cystine, 3.20 of valine, 2.50 of methionine, 0.85 of tryptophan, 3.50 of phenylalanine, 2.50 of isoleucine, 3.40 of leucine, 5.50 of lysine acetate, 2.90 of proline, 0.10 of cysteine hydrochloride and 0.20 of lemon acid. The composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test, a test result shows that the pharmaceutical composition containing 18 amino acids is as stable as or more stable than like products which are sold in the markets and contain sulfites.
Owner:郑飞雄
Medicine composition containing 15 kinds of amino acid and preparation method thereof
ActiveCN101357118ASolve the problem of trace oxygenSolve the problem of oxygen incorporationOrganic active ingredientsPharmaceutical delivery mechanismArginineTryptophan
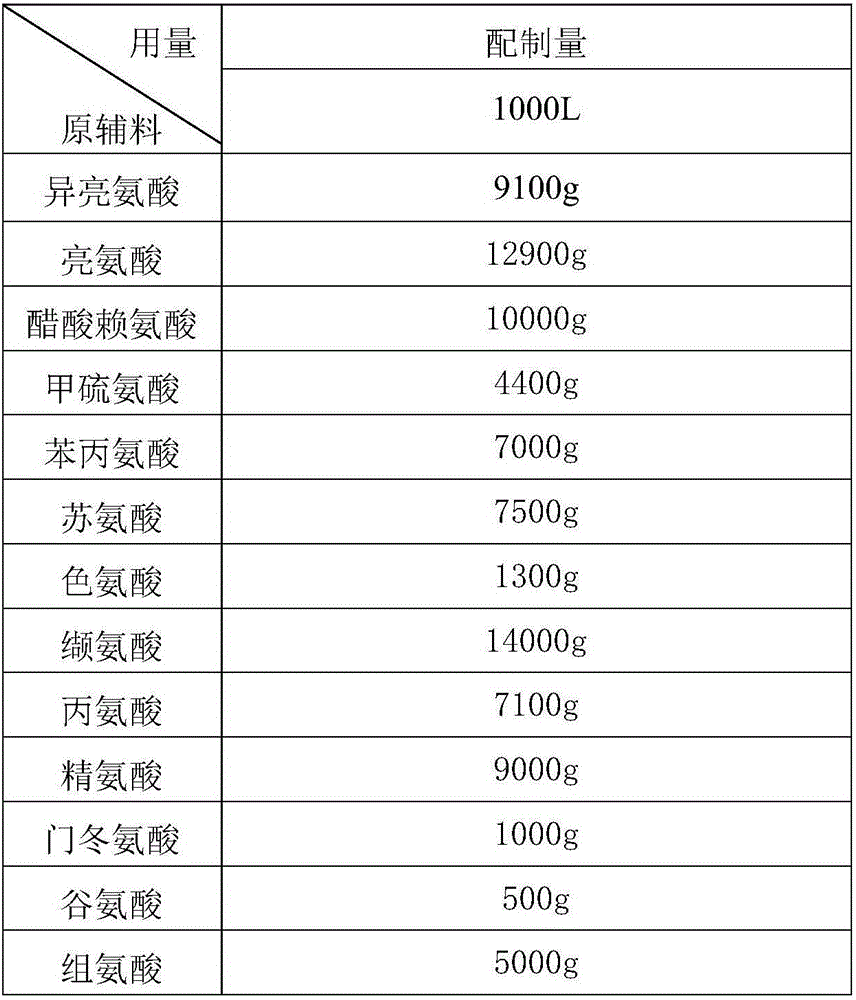
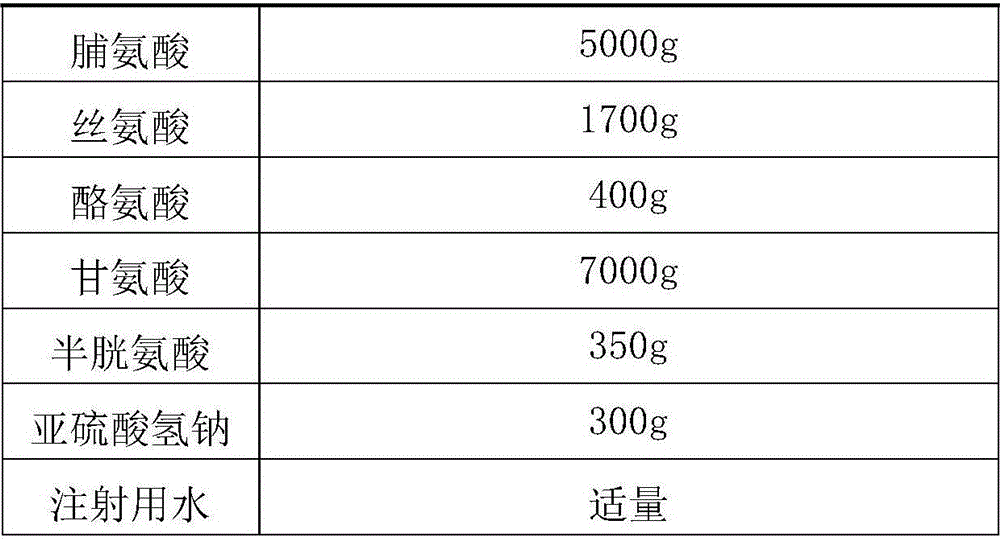
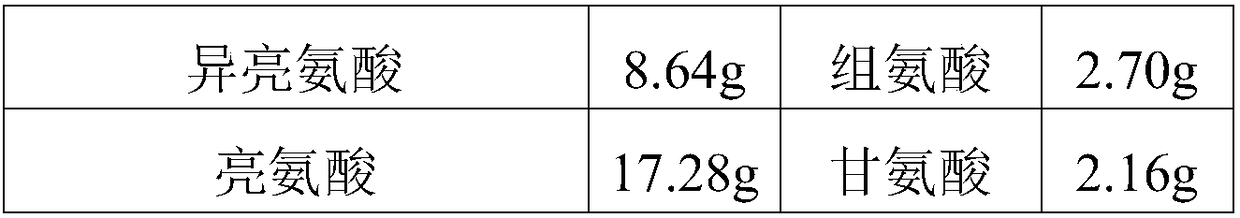
The invention discloses a medicine combination which contains 15 amino acids and the preparation method thereof; the medicine combination is characterized in that a compound amino acid injecta with different concentrations are prepared by the 15 amino acids which serve as raw materials and admixture according to the following parts by weight: 6.1-10.8 parts of L-Isoleucine, 8.8-16.6 parts of L-Leucine, 4.6-10.4 parts of L-Lysine Acetate, 0.8-3.0 parts of L-Methionine, 0.8-3.9 parts of L-Phenylalanine, 1.6-5.4 parts of L-threonine, 0.5-1.1 parts of L-Tryptophan, 6.7-10.7 parts of L-Valine, 3.2-9.3 parts of L-alanine, 4.6-7.2 parts of L-arginine, 1.2-2.9 parts of L-Histidine, 5.0-9.6 parts of L-proline, 2.6-6.0 parts of L-serine, 2.6-10.8 parts of glycin, 0.1-1.0 parts of L-Cysteine hydrochloride, 0.1-0.5 parts of citric acid and moderate water for injection. The injecta does not contain sulphite type chemical inhibitor, thoroughly solves the harm of sulphite type on human body, and ensures that the obtained products are safer. The PH value of the injecta is 5.5-7.0. By the accelerated test and quality test, the results show that the stability of the medicine combination which contains 15 amino acids is the same as or better than like products which contains sulphite type sold on market.
Owner:郑飞雄
Alfalfa seed obducens agent and production process thereof
InactiveCN104496662AThe production application effect is remarkableReduce applicationsAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersMonopotassium phosphateGibberellin
The invention discloses an alfalfa seed obducens agent which is prepared from the following raw materials in percentage by weight: 1.5-5% of carboxymethylcellulose, 0.5-3% of rhizobium of alfalfa, 0.5-7% of biological humic acid, 0.2-3% of urea, 0.2-3% of monopotassium phosphate, 0.1-0.7% of ammonium molybdate, 0.1-0.5% of borax, 2-6% of sulfur powder, 0.15-0.7% of ferrous sulfate, 5-8% of sodium polyacrylate, 0.01-0.05% of indolebutyric acid, 0.01-0.05% of gibberellin, 0.01-0.05% of lysine acetate, 1.0-8.0% of thiram, 2.0-8.5% of thiophanate-methyl and the balance of alfalfa seeds. Aiming to the characteristics that alfalfa in the seedling stage is not saline-alkaline tolerant, draught tolerant, susceptible to diseases and the like, anti-salt and alkali, anti-drought and soil sterilizing factors are respectively added into the obducens agent disclosed by the invention, and moreover, the alfalfa seed obducens agent is obvious in production and application effects and is deeply welcomed by farmer households.
Owner:酒泉大业种业有限责任公司
Aescin medicine composition and its prepn process and use
ActiveCN1931176ALess irritatingEliminate side effectsOrganic active ingredientsAntipyreticIrritationDrug carrier
The present invention relates to aescin medicine composition and its preparation process and use. The aescin product of purity over 90 % may be compounded with medicine carrier to form composition with obvious synergistic effect, reduced stimulation of aescin on blood vessels and muscles, and obviously raised effects of diminishing inflammation, antagonizing effusion, improving blood circulation, etc. The composition features its less stimulation, high safety, high treating index, etc.
Owner:WUHAN AIMIN PHARMA
Amino acid injection and preparation method thereof
ActiveCN101401785APain reliefReduce clinical adverse reactionsOrganic active ingredientsMetabolism disorderAntioxidantThreonine
The invention discloses an amino acid injection, commonly known as propranolol, which relates to a pharmaceutical preparation containing 18 kinds of amino acids. The amino acid injection contains the following components per 1,000 milliliters: isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, valine, glycine, alanine, glutamic acid, aspartic acid, proline, serine, cystine, lysine hydrochloride or lysine acetate, arginine hydrochloride or arginine, histidine hydrochloride or histidine, tyrosine or acetyl tyrosine, and sodium bisulfite or sodium meta-bisulphite. The osmotic pressure ratio of the amino acid injection is lower than 1.8, which effectively reduces the pain for a patient in infusion; the added quantity of antioxidant is small, which can reduce the clinical adverse reactions; and an infusion bottle body is large, which is convenient to mix liquid in clinical application.
Owner:GUANGDONG LITAI PHARM CO LTD
Formula of liposome preparation containing compound amino acid and preparation method thereof
ActiveCN102526032AStrong comfortKeep moistOrganic active ingredientsPeptide/protein ingredientsVitamin CArginine
The invention discloses a formula of a liposome preparation containing compound amino acids and a preparation method thereof; a raw material mass ratio of the liposome preparation is determined; the preparation method comprises the following steps: (1) weighing soybean phosphatide and cholesterol, adding water, heating and stirring to prepare an oil phase; (2) weighing cysteine hydrochloride and tryptophan, adding process water for dissolution, orderly adding some or all of valine, isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, arginine, glycine, and praline; then orderly adding one or more than one of auxiliary materials of vitamin A, vitamin C, vitamin E, and vitamin K, and a film forming material, finally adding potassium sorbate or ethylparaben, stirring toprepare a water phase; (3) mixing the oil phase and the water phase, shearing by a high-speed shearing machine to obtain the liposome preparation. The invention initiates the technology for preparingliposome without the adoption of any organic solvents; the prepared liposome preparation has high entrapment efficiency, and good stability; and industrial production is realized.
Owner:GUIZHOU YANGSHENG MEDICAL INSTR
Compound alpha-keto acid tablets and preparation method and detection method thereof
ActiveCN102961378AEffective treatment effectAvoid product qualityPeptide/protein ingredientsComponent separationThreonineTyrosine
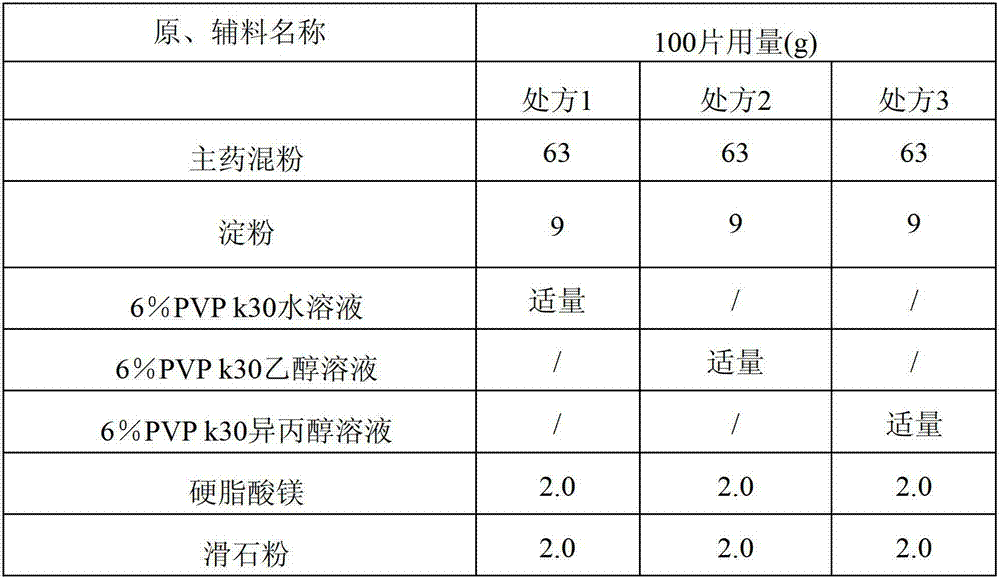
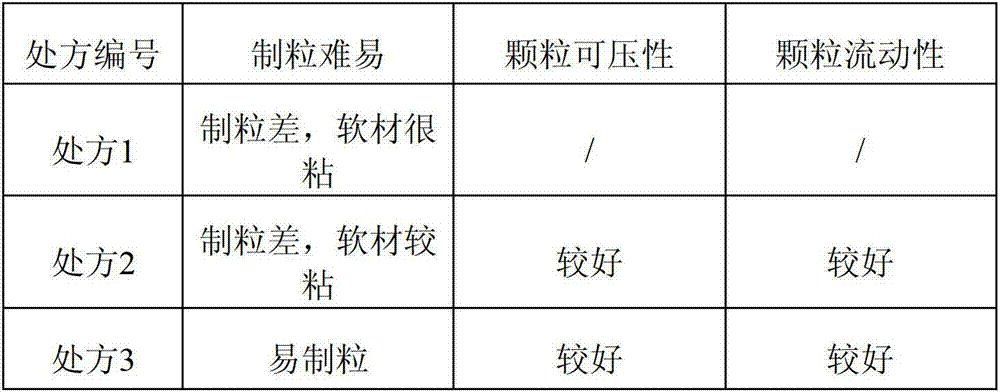
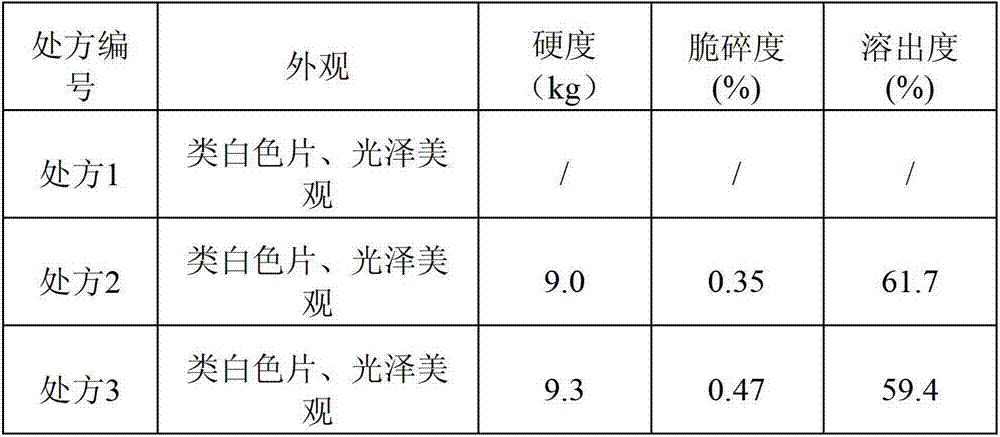
The invention discloses compound alpha-keto acid tablets and a preparation method and a detection method thereof. The preparation method comprises the following steps of: taking calcium 3-methyl-2-oxovalerate, ketoleucine calcium, alpha-ketophenylalanine calcium, keto-valine-calcium, D,L-alpha-hydroxymethionine calcium, lysine acetate, threonine, tryptophan, histidine and tyrosine, crushing the main medicaments respectively and sieving with a sieve of 100 meshes; sieving starch, talc powder and magnesium stearate and sieving with a sieve of 80 meshes respectively; mixing the main medicaments and the starch uniformly, preparing a soft material from 6 percent polyvinyl pyrrolidone (PVP) k30 isopropanol solution, granulating with a sieve of 20 meshes, and drying at the temperature of 40 DEG C; reshaping, adding magnesium stearate, the talc powder and crosslink povidone, and mixing uniformly; detecting an intermediate, and tabletting; and coating, and thus obtaining the compound alpha-keto acid tablets. The tablets with excellent granule pressing property, flowability, hardness and dissolution degree can be prepared by using the method, and the detection method can further ensure the quality of the prepared compound alpha-keto acid tablets.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Methods to inhibit infection during wound healing with topical compositions including amino acids
ActiveUS20160106715A1Avoid woundsBiocidePeptide/protein ingredientsHistidine Monohydrochloride MonohydrateArginine
A method that inhibits infection during wound healing by topical application of a single composition, which contacts a wound for a period of time to initiate wound healing. The single composition may include a mixture of all or some of the amino acids including: histidine monohydrochloride monohydrate; isoleucine; leucine; lysine acetate; methionine; phenylalanine; threonine; tryptophan; valine; alanine; arginine acetate; aspartic acid; glutamine; glycine; proline; serine; and tyrosine, which may provide aqueous mixtures of a range of osmolarities or a powdered mixture of amino acids. The single composition may also include any of a carbohydrate, a mixture of fatty acids, vitamins, and mineral nutrients, and another mixture of amino acid derivatives and preservatives. The number of moles of the carbohydrate is less than and proportional to a sum of the number of moles of any mixture of amino acids in the single composition.
Owner:PLUMB JOHN H
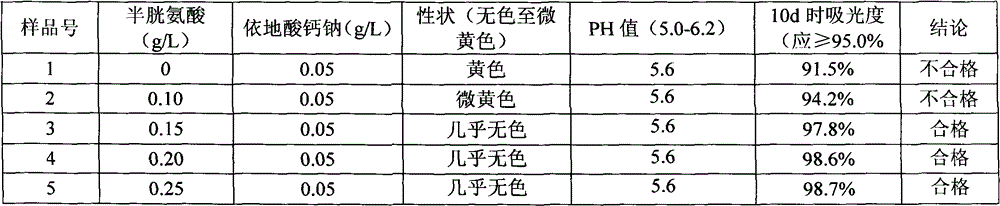
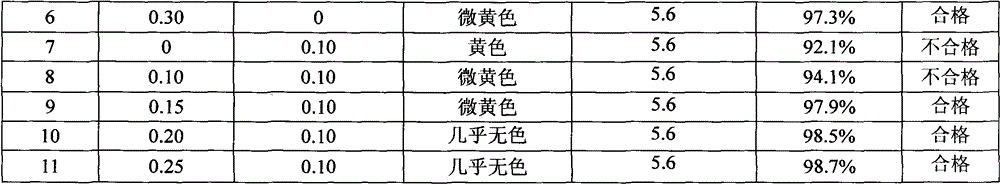
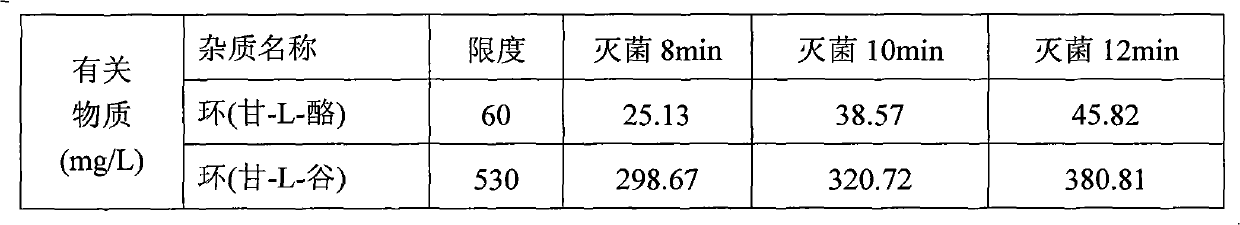
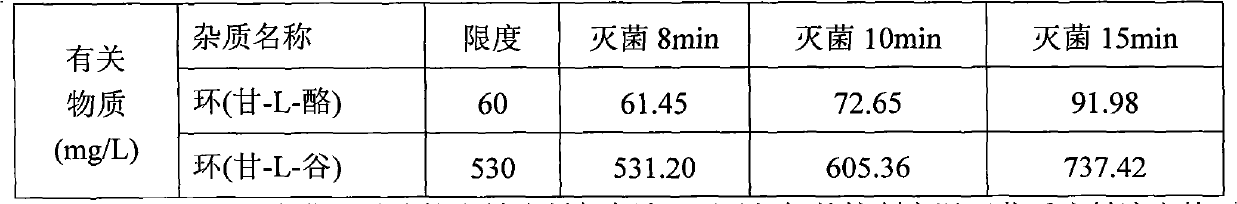
Method for assaying related substances in lysine acetate by high-performance liquid chromatography
InactiveCN105606724AEfficient separationHigh precisionComponent separationMethanol waterMonosodium phosphate
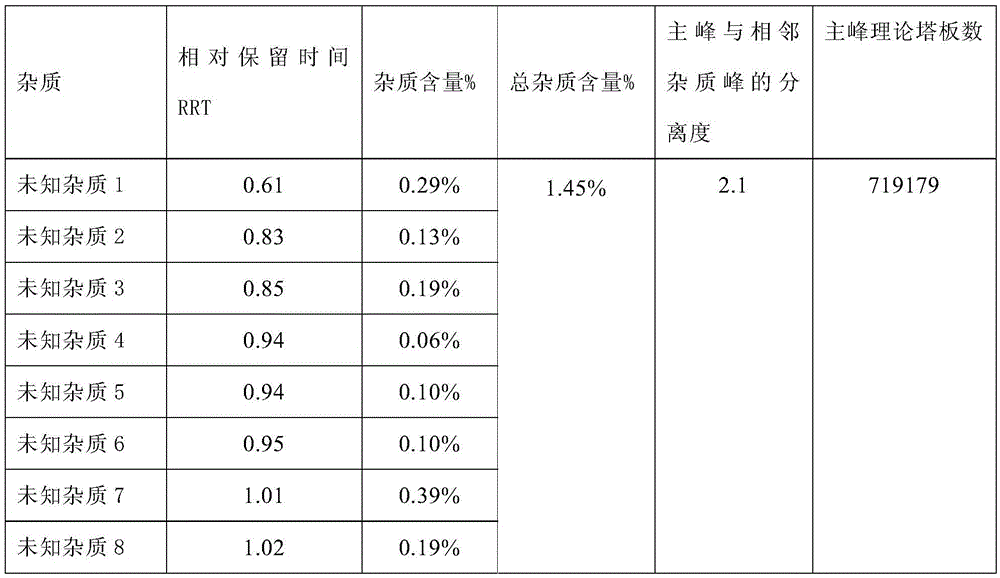
The invention relates to a method for assaying related substances in lysine acetate by high-performance liquid chromatography. According to the method, by taking octadecylsilane bonded silica gel as a chromatographic column and taking a sodium dihydrogen phosphate buffer solution and methanol-water as mobile phases, the contents of the related substances in the lysine acetate can be assayed, so that the masses of the related substances in the lysine acetate can be effectively controlled.
Owner:SHANGHAI JINGFENG PHARMA
Pharmaceutical composition containing 17 amino acids
ActiveCN102552251AWidely used clinical populationMeet registration requirementsOrganic active ingredientsMetabolism disorderArginineTyrosine
The invention relates to a pharmaceutical composition containing 17 amino acids. The pharmaceutical composition comprises the following raw materials and auxiliary materials: proline, serine, alanine, isoleucine, leucine, tyrosine, glutamic acid, phenylalanine, arginine, lysine acetate, valine, threonine, histidine, tryptophan, methionine, acetylcysteine, glycine, sorbitol, and sodium bisulfite. The product is free of chlorine ion, and is wider in application crowd range; in the production, an acid or a base is not additionally added for adjusting the PH value; and the product is prepared according to the component ratio, and has the PH value of 5.5-7.0. Therefore, the pharmaceutical composition meets the pharmaceutical registering requirements that the material consumption is reduced andthe production cost is lower, and has equivalent or better quality and stability as compared with the commercially available products.
Owner:湖北长联杜勒制药有限公司
Composition containing eighteen amino acids
ActiveCN102743379AAvoid side effectsAvoid Hyperchloric AcidosisOrganic active ingredientsPeptide/protein ingredientsArginineAntioxidant
The invention provides a composition containing eighteen amino acids and a preparation method of the composition. The composition is a composite amino acid injection solution 18AA-II and comprises the following components by weight: 1.5-3.3g of aspartic acid, 2.5-5.7g of glutamic acid, 1.9-4.5g of serine, 3.0-6.8g of histidine, 3.5-7.9g of glycine, 2.5-5.7g of threonine, 7.2-16.3g of alanine, 4.9-11.2g of arginine, 0.2-0.3g of tyrosine, 0.16-0.2g of cystine, 3.2-7.3g of valine, 2.5-5.7g of methionine, 0.85-1.9g of tryptophane, 3.5-7.9g of phenylalanine, 2.5-5.7g of isoleucine, 3.4-7.9g of leucine, 5.5-12.7g of lysine acetate, 2.9-6.8g of proline, 1.3-2.75ml of glacial acetic acid, 0.2g of cysteine and 0.05g of calcium disodium edetate. The composition does not contain a sulfite antioxidant so that the obtained product can be more safely used clinically.
Owner:武汉天安医药科技有限公司
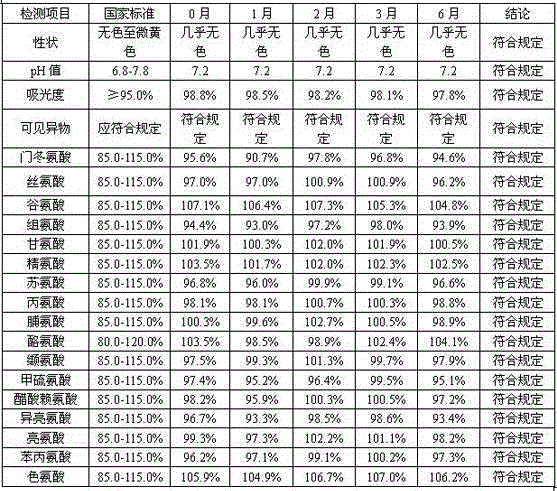
Method for producing lysine acetate for injection
ActiveCN102229540AIncrease productivityEasy to separateOrganic compound preparationAmino-carboxyl compound preparationNeutral Amino AcidsFiltration membrane
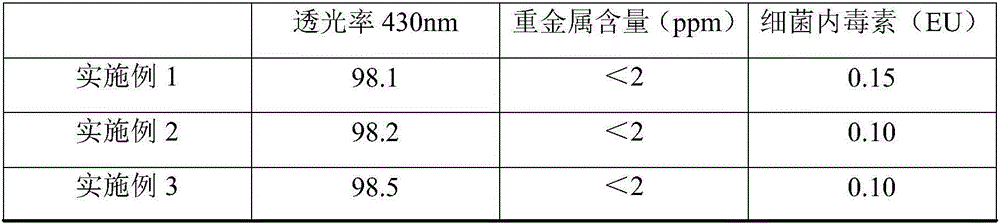
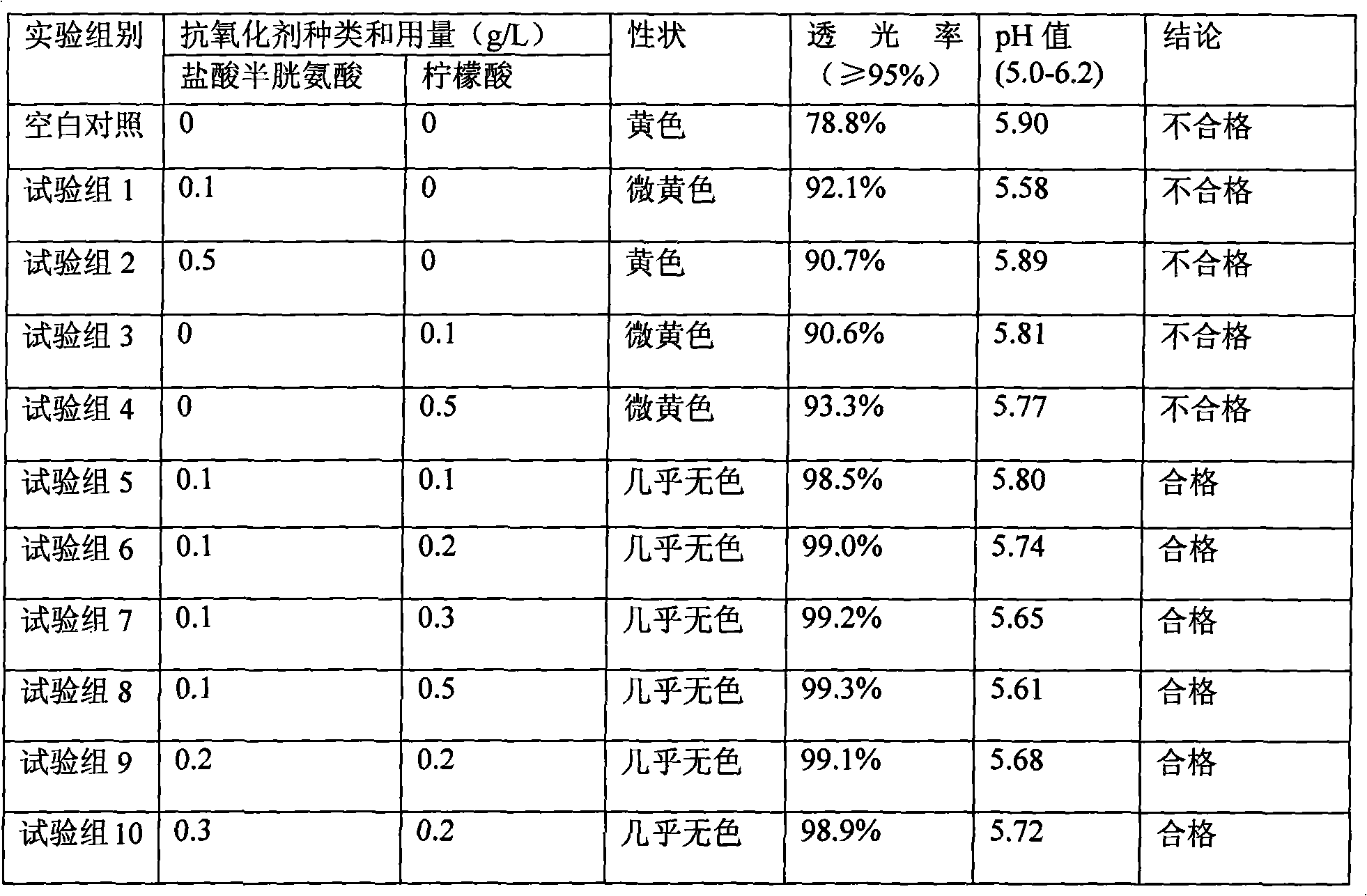
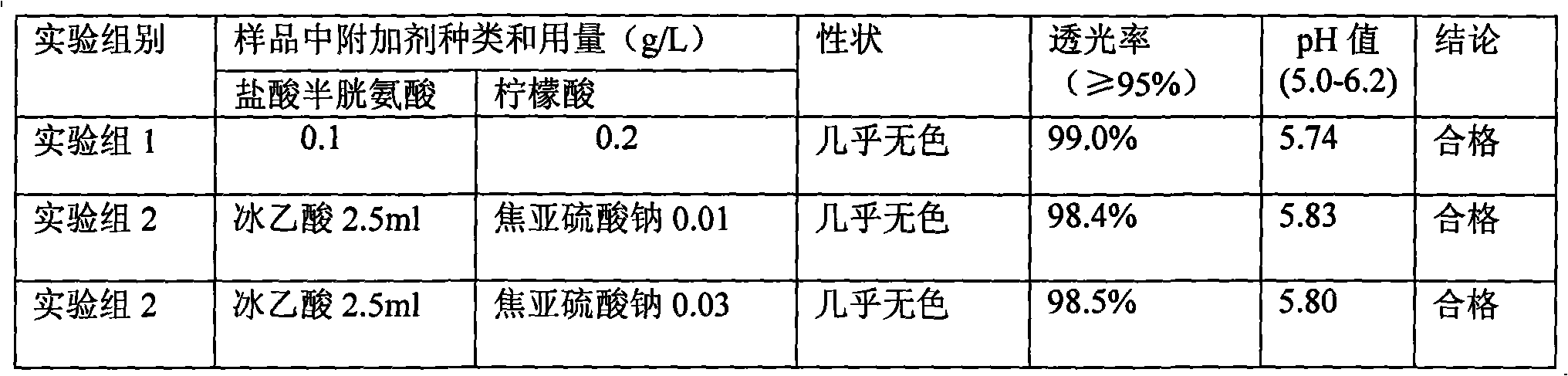
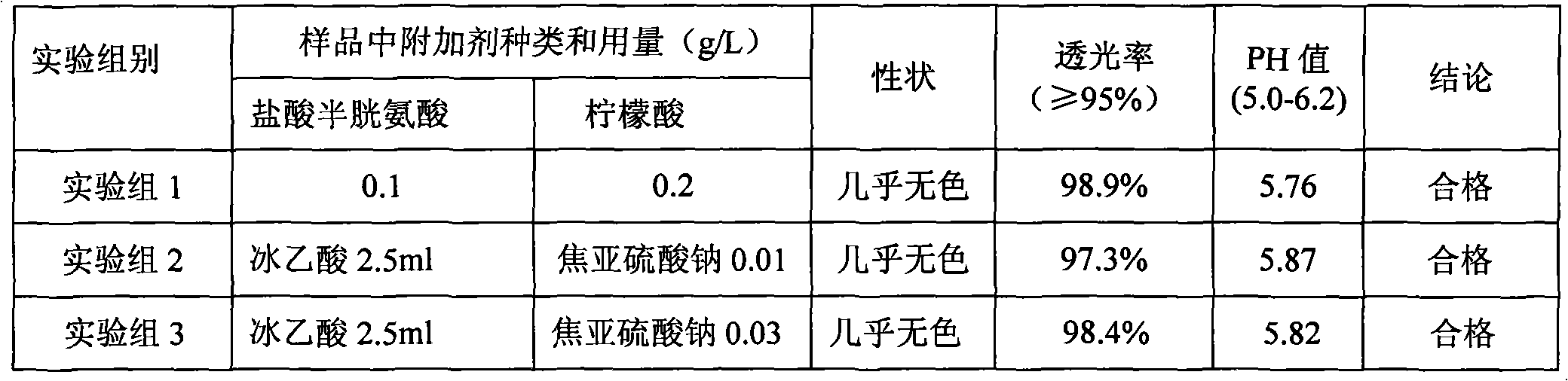
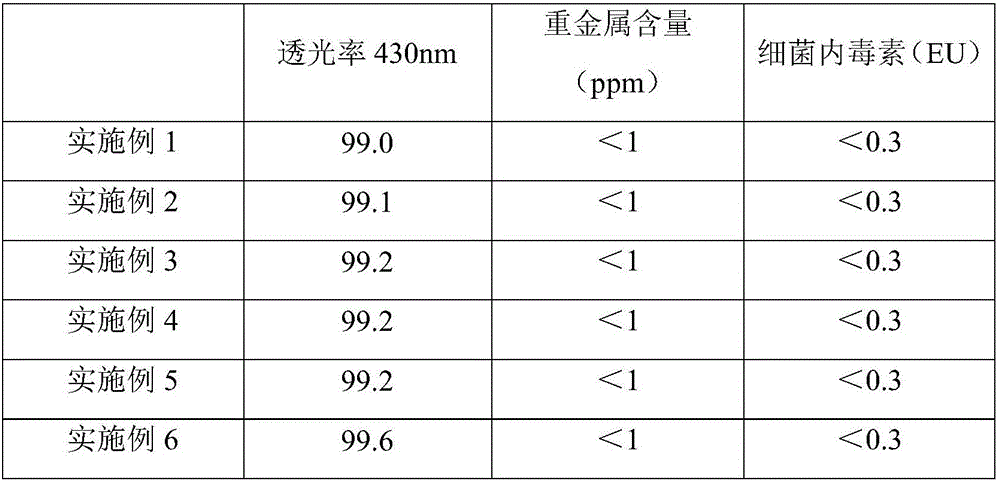
The invention discloses a method for producing lysine acetate for injection, comprising the following steps of: preparing industrial hydrochloric acid lysine into aqueous solution; after adsorbing and saturating through balanced hole strong acid cation exchange resin; using 0.01-0.05 mol / L of ammonia water to elute neutral amino acid heteroacid; then, using 0.5-1 mol / L of ammonia water to elute histidine; finally, using 1.5-5 mol / L of ammonia water to elute lysine; filtering through an ultra-filtration membrane; filtering through a nano-filtration membrane; concentrating and crystallizing; and obtaining the lysine acetate for injection. The better separation effect and production efficiency can be achieved. The contents of the amino acid heteroacid and bacterial endotoxin in the product are very low. The light transmission rate of the solution is greatly improved. The purity of the product can reach above 99.8%. The quality exceeds the quality standard for injection of Chinese pharmacopoeia.
Owner:汕头市佳禾生物科技有限公司
Compound amino acid injection and preparation method thereof
InactiveCN102940628AGood metal ion chelating performanceInhibition of catalysisOrganic active ingredientsMetabolism disorderAntioxidantArginine
A compound amino acid injection provided by the invention comprises the following solvents in every 1000 mL: 11.6-14.2g of leucine, 6.8-8.3g of threonine, 8.2-10.0g of isoleucine, 6.3-7.7g of glycine, 12.6-15.4g of valine, 6.3-7.7g of phenylalanine, 1.2-1.4g of tryptophan, 4.0-4.8g of methionine, 0.4-0.6g of glutamic acid, 6.4-7.8g of alanine, 1.5-1.9g of serine, 4.5-5.5g of proline, 0.35-0.45g of tyrosine, 9.0-11.0g of lysine acetate, 8.1-9.9g of arginine, 4.5-5.5g of histidine, 0.9-1.1g of aspartate, 0.3-0.4g of cysteine, and 0.04-0.06g of calcium disodium edetate. The injection does not contain sulfite antioxidant, and employs cysteine and sodium calcium edetate as antioxidants and metal chelators; and nitrogen is used for protection in a production process, so as to reduce oxygen content in solution as much as possible and produce safer product for clinical usage.
Owner:湖北长联杜勒制药有限公司
Compound amino acid injection (18AA-I) composition
ActiveCN102988359AReduce the content of antihypertensive substancesQuality improvementOrganic active ingredientsPeptide/protein ingredientsArginineThreonine
The invention relates to a compound amino acid injection (18AA-I) composition. Each 1000ml of the compound amino acid injection composition contains 2.8-3.5g of isoleucine, 3.5-4.0g of serine, 1.7-2.5g of glycine, 6.0-6.5g of lactamine, 1-1.5g of methionine, 1-1.5g of tryptophan, 6.5-7.5g of leucine, 3.7-4.3g of aspartic acid, 0.5-1.0g of N-acetyl-L-tyrosine, 5.2-5.8g of proline, 2.5-3.0g of phenylalanine, 3.1-3.8g of valine, 7.7-8.3g of lysine acetate, 6.8-7.3g of glutamic acid, 1.7-2.5g of histidine, 3.7-4.5g of arginine, 3.4-4.0g of threonine and 0.5-1.5g of cysteine hydrochloride and is characterized in that each 1000ml of the injection further contains 0.5-1g of lactic acid.
Owner:TIANJIN JINYAO GRP
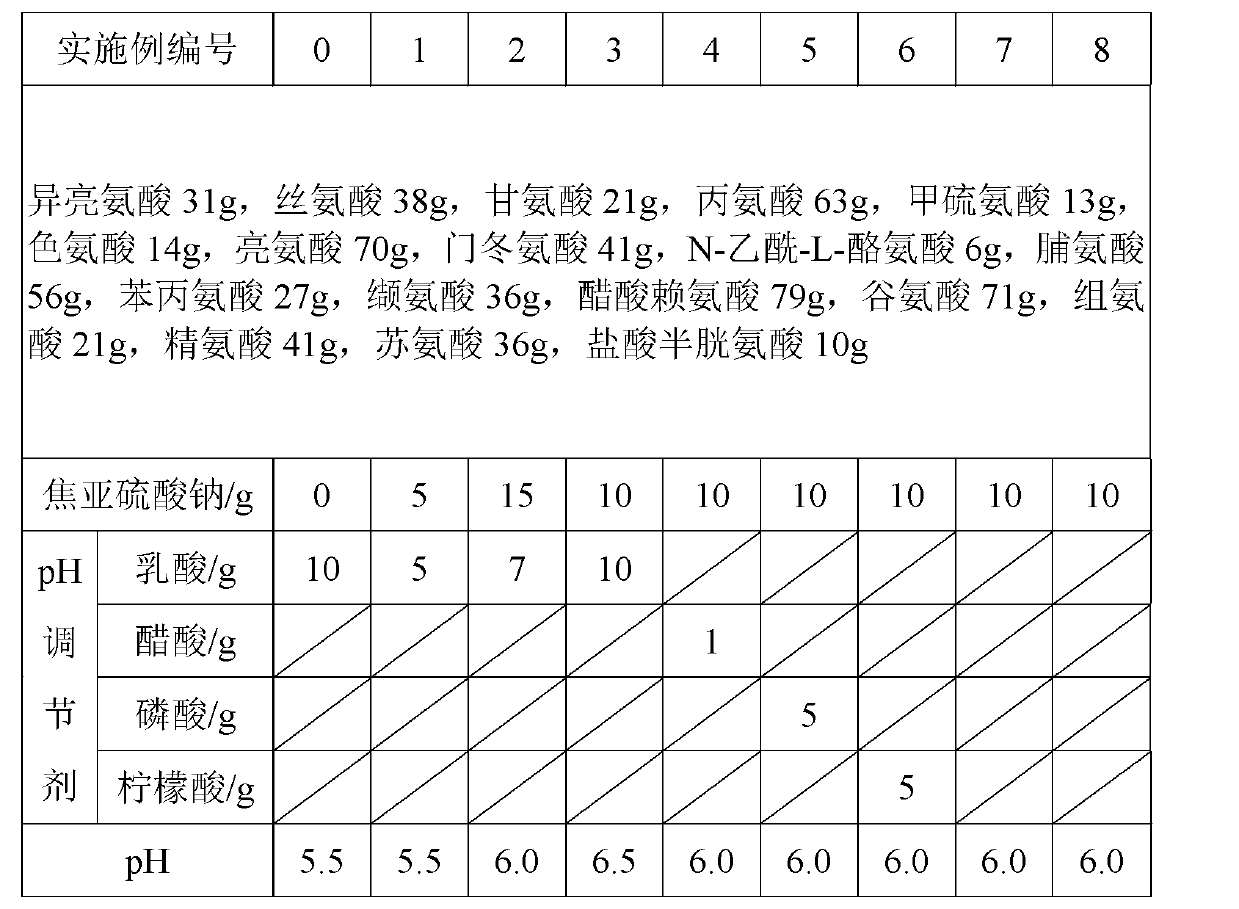
Preparation method of compound amino acid (15) dipeptide (2) injecta
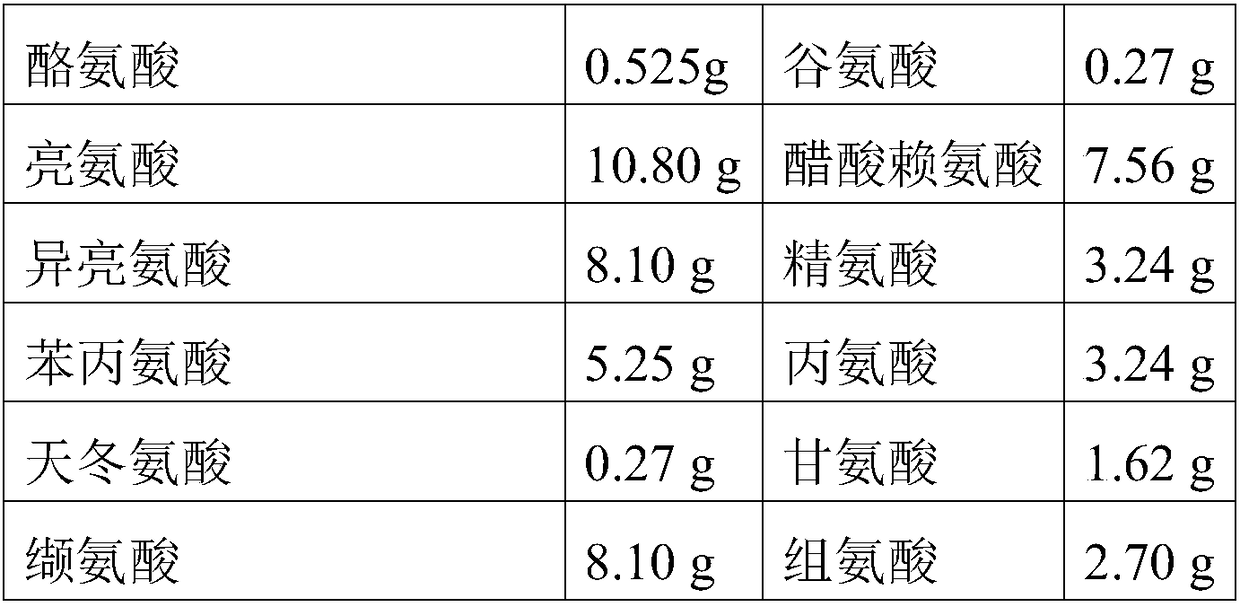
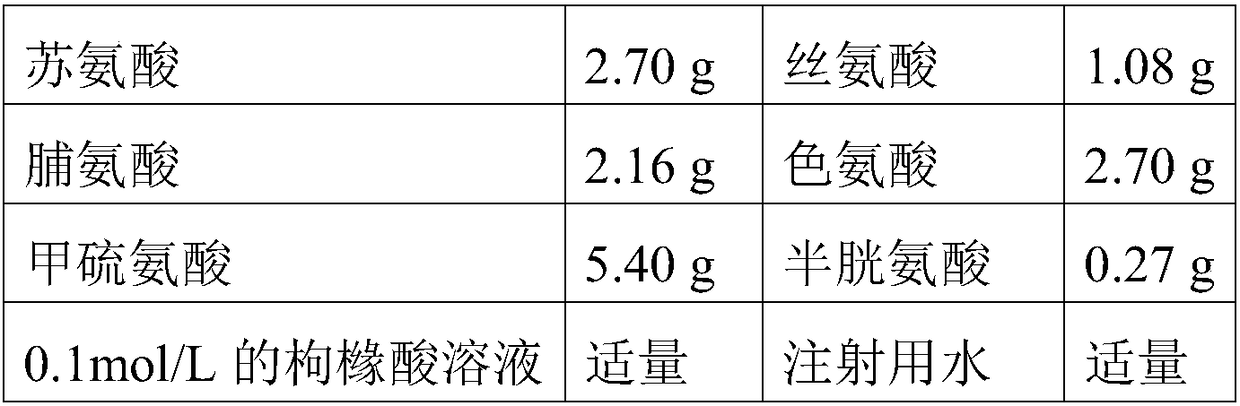
The invention relates to a preparation method of compound amino acid (15) dipeptide (2) injecta, specifically comprising the following steps of: under the whole-course protection of nitrogen, taking water for injection with a certain quantity, sequentially adding glycyl-L-glutamine, glycyl-L-tyrosine and arginine with prescription quantity, stirring and dissolving, clearly dissolving, adding aspartate, glutamic acid, leucine, isoleucine and phenylalanine with prescription quantity which is not less than 60 screen meshes, dissolving and clearly dissolving, adding alanine, histidine, L-lysine monoacetate, methionine, proline, serine, threonine, tryptophan and valine with prescription quantity, stirring and clearly dissolving, adjusting the pH at 5.4-5.8 by citric acid, adding full dose of 0.10% (w / v) of activated carbon, stirring for 30min under the temperature of 60DEG C, fixing the volume to total volume by the water for injection after filtering in a decarbonizing way, sieving by a filter membrane with 0.22 micrometers, filling, charging nitrogen, adding a plug, pricking an aluminum cap, and sterilizing for 8-12min in a hot-press way under the temperature of 121DEG C (F0 value is larger than 8), wherein the quality of the injecta can achieve the quality standard of an imported drug of the finished product of the German Fresenius Corporation.
Owner:北京紫萌医药科技有限公司
Compound amino acid injection (18AA-IV) composition
InactiveCN102973556AGood storage stabilityLong validity periodOrganic active ingredientsPeptide/protein ingredientsArginineTyrosine
The invention discloses a compound amino acid injection (18AA-IV) composition, each 1000ml of which comprises 4.0-4.5g of leucine, 1.5-2.0g of isoleucine, 4.0-4.5g of lysine acetate, 1.0-1.5g of methionine, 1.8-2.2g of histidine, 0.10-0.15g of tyrosine, 1.8-2.3g of alanine, 3-4g of glycine, 1.0-1.5g of aspartate, 2.0-2.5g of glutamic acid, 1.0-1.5g of proline, 3.0-3.5g of phenylalanine, 2.0-2.5g of threonine, 0.3-0.8g of N-acetyl L-acetyl, 1-2g of valine, 0.5-1g of serine, 2-3g of arginine, 0.2-0.7g of cysteine hydrochloride and 70-80g of glucose. The compound amino acid injection (18AA-IV) composition is characterized by containing 0.5-5g of citric acid per 1000ml.
Owner:TIANJIN JINYAO GRP
Notoginsenoside pharmaceutical composition and method for preparing the same and use thereof
The present invention relates to medicine composition of aescin and its preparation process and use. The aescin with purity over 90 % may be compounded with medicine carrier to form medicine composition with high synergistic effect, obviously reduced irritation on blood vessel and muscle, obviously raised medicinal effects of resisting inflammation, resisting exudation, improving blood circulation, etc. The medicine composition has low irritation, high safety, high therapeutic index and other features.
Owner:WUHAN AIMIN PHARMA
Preparation method of compound amino acid injection 18AA
InactiveCN106361749AStable in natureExtended storage timeOrganic active ingredientsPeptide/protein ingredientsArginineTyrosine
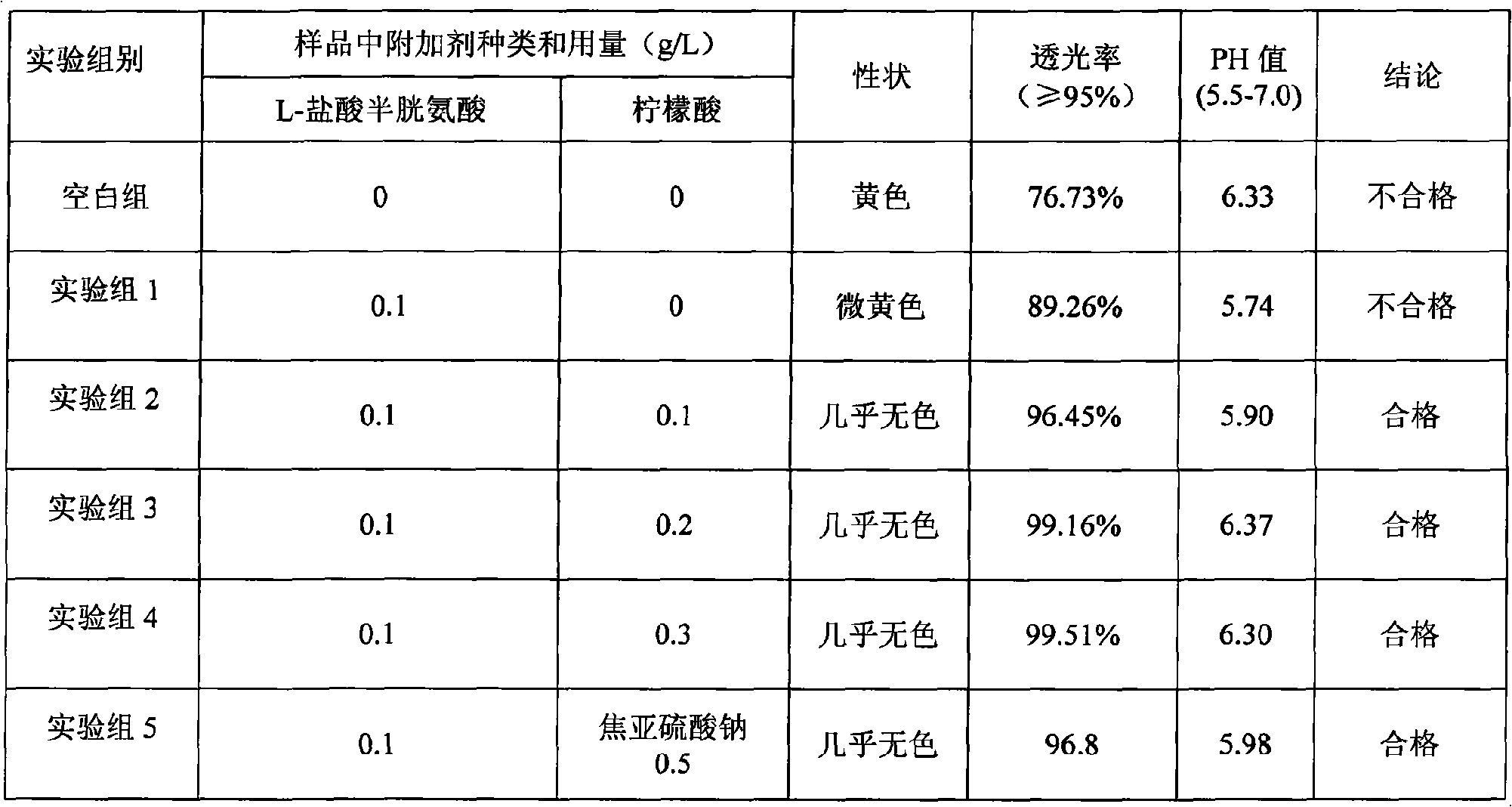
The invention relates to a preparation method of a compound amino acid injection 18AA. The method includes the following steps that firstly, infusion bottles are washed and then conveyed to a filling room; secondly, arginine is stirred to be dissolved, and then valine, phenylalanine, alanine, aspartic acid, proline, serine, glutamic acid, glycine, tyrosine, isoleucine, leucine, methionine, threonine and histidine are added in sequence and stirred to be dissolved; thirdly, the solution is cooled to 30-50 DEG C, lysine acetate, tryptophan, cysteine and a sodium bisulfite solution are added, and after stirring is carried out for dissolution, water for injection is supplemented to a full dose; fourthly, after capping and sealing are carried out, sterilization is carried out. In this way, full dissolution of the components is promoted, and the light transmittance of the compound amino acid injection 18AA is guaranteed; besides, the property of the compound amino acid injection is stable, long-time storage is easy, and absorption is promoted.
Owner:安徽富邦药业有限公司
Preparation method of medicine grade lysine acetate
ActiveCN102070477AShort processEasy to operateOrganic compound preparationAmino-carboxyl compound preparationActivated carbonAcetic acid
The invention provides a preparation method of medicine grade lysine acetate, which comprises the following steps: 1) enabling a biological fermentation broth containing L-lysine to be reacted with acetic acid, adding activated carbon into reaction solution for performing decolorization at least once, then concentrating, crystallizing and getting a lysine acetate crude product; and 2) adding the lysine acetate crude product obtained in the step 1) into distilled water for dissolution, performing the decolorization at least once, and further performing fine filtration, concentration and crystallization. Various indicators of the medicine grade lysine acetate prepared according to the invention are in line with the pharmacopoeia standard, the preparation of the medicine grade lysine acetate by using the lysine fermentation broth has no harsh reaction conditions and is applicable to industrial production, and the preparation of the medicine grade lysine acetate by using the lysine fermentation broth which is relatively low in price can realize value appreciation to the maximum extent.
Owner:BENGBU BBCA MEDICINE SCI DEV
Compound amino acid injection (18AA-IV) composition
InactiveCN102988358AReduce the content of antihypertensive substancesQuality improvementOrganic active ingredientsPeptide/protein ingredientsArginineThreonine
The invention relates to a compound amino acid injection (18AA-IV) composition. Each 1000ml of the compound amino acid injection composition contains 4.0-4.5g of leucine, 1.5-2.0g of isoleucine, 4.0-4.5g of lysine acetate, 1.0-1.5g of methionine, 1.8-2.2g of histidine, 0.10-0.15g of tyrosine, 1.8-2.3g of alanine, 3-4g of glycine, 1.0-1.5g of aspartic acid, 2.0-2.5g of glutamic acid, 1.0-1.5g of proline, 3.0-3.5g of phenylalanine, 2.0-2.5g of threonine, 0.3-0.8g of N-acetyl-L-tyrosine, 1-2g of valine, 0.5-1g of serine, 2-3g of arginine, 0.2-0.7g of cysteine hydrochloride and 70-80g of glucose and is characterized in that each 1000ml of the injection further contains 0.5-1g of lactic acid.
Owner:TIANJIN JINYAO GRP
Compound amino acid dipeptide injection and preparation method thereof
InactiveCN110693827ASpeed up recovery timeExcellent wound healingOrganic active ingredientsPharmaceutical delivery mechanismGlycyltyrosineArginine
The invention discloses a compound amino acid dipeptide injection. The compound amino acid dipeptide injection is prepared from the following components in pats by weight: 4-8 parts of alanine, 2-6 parts of arginine, 0.7-2.7 parts of aspartic acid, 0.9-1.3 parts of glutamic acid, 1-5 parts of histidine, 0.9-1.3 parts of isoleucine, 1.6-5.6 parts of leucine, 2.5-6.5 parts of lysine acetate, 0.9-1.3parts of methionine, 1.2-5.2 parts of phenylalanine, 1-5 parts of proline, 0.8-2.8 parts of serine, 0.9-1.3 parts of threonine, 0.3-2.3 parts of tryptophan, 0.85-1.35 parts of valine, 8-16 parts of glycylglutamine, 0.75-2.75 parts of glycyltyrosine, 0.5-2.5 parts of a PH regulator, 20-38 parts of water and 0.1-0.3 part of an adsorbent. The invention further provides a preparation method of the compound amino acid dipeptide injection. Glutamine is generated by the glycyltyrosine, the generated glutamine repairs a postoperative wound, compound amino acid has a better effect of promoting wound healing, the healing time of a postoperative patient is shortened, and the healing promoting effect is better.
Owner:天津金耀集团湖北天药药业股份有限公司
Pharmaceutical composition containing 19 types of amino acids and preparation method thereof
ActiveCN109381423AAvoid harmEasy to prepareMetabolism disorderPharmaceutical delivery mechanismArginineTyrosine
The invention discloses a pharmaceutical composition containing 19 types of amino acids and a preparation method thereof. The pharmaceutical composition comprises tyrosine, leucine, isoleucine, phenylalanine, aspartic acid, valine, threonine, proline, methionine, glutamic acid, lysine acetate, arginine, alanine, glycine, histidine, serine, tryptophan, cysteine, taurine, a pH value conditioning agent, and water. The pharmaceutical composition can be free of sulfites, thereby completely eliminates the safety hazards of sulfites to the human body, and makes the clinical use safer; and the expiration period of the composition is longer.
Owner:湖北一半天制药有限公司 +1
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439031BSolve the problem of trace oxygenSolve the oxygen redissolution problemOrganic active ingredientsMetabolism disorderArginineAntioxidant
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-II) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 1.50 of aspartic acid, 2.50 of glutamic acid, 1.90 of serine, 3.00 of histidine, 3.50 of glycine, 2.50 of threonine, 7.20 of alanine, 4.90 of arginine, 0.20 of tyrosine, 0.20 of cystine, 3.20 of valine, 2.50 of methionine, 0.85 of tryptophan, 3.50 of phenylalanine, 2.50 of isoleucine, 3.40 of leucine, 5.50 of lysine acetate, 2.90 of proline, 0.10 of cysteine hydrochloride and 0.20 of lemon acid. The composition does not contain a sulfite antioxidant so that the pharmaceutical composition isclinically used in a safer manner. After an accelerated test, a test result shows that the pharmaceutical composition containing 18 amino acids is as stable as or more stable than like products whichare sold in the markets and contain sulfites.
Owner:郑飞雄
Compound amino acid injection (18AA-III) composition
InactiveCN102973557AReduce the content of antihypertensive substancesQuality improvementOrganic active ingredientsMetabolism disorderArginineTyrosine
The invention relates to a compound amino acid injection (18AA-III) composition. Each 1000ml of compound amino acid injection (18AA-III) composition contains 5.0-6.0g of isoleucine, 7.5-8.0g of arginine, 12-13g of leucine, 3.5-4.0g of aspartic acid, 12-13g of l-Lysine acetate salt, 0.8-1.5g of cysteine, 3.0-4.0g of methionine, 6.0-7.0g of glutamic acid, 9.0-9.5g of phenylalanine, 5.5-6.5 of histidine, 6.0-7.0 of threonine, 3.0-3.5g of praline, 1-1.5g of trytophan, 2-2.5g of serine, 4-5g of valine, 0.1-0.5g of tyrosine, 6.0-6.5g of alanine and 10-11g of glycine. The compound amino acid injection (18AA-III) composition is characterized in that each 1000ml of injection contains 0.5-1g of lactic acid.
Owner:TIANJIN JINYAO GRP
Compound amino acid solution for tree nutrient solution and preparation process thereof
The invention provides a compound amino acid solution for a tree nutrient solution and a preparation process thereof. Specifically, the compound amino acid solution for the tree nutrient solution comprises the following components by mass: 1000 parts of deionized water, 0.20-0.30 part of tyrosine, 4.80-5.00 parts of leucine, 3.48-3.56 parts of isoleucine, 3.50-3.70 parts of valine, 2.21-2.29 parts of methionine, 1.92-2.08 parts of alanine, 5.23-5.43 parts of phenylalanine, 0.71-0.79 part of glutamic acid, 2.42-2.58 parts of aspartic acid, 4.22-4.36 parts of lysine acetate, 2.42-2.58 parts of threonine, 7.54-7.66 parts of glycine, 0.90-1.10 parts of proline, 0.90-1.10 parts of serine, 4.82-5.12 parts of arginine hydrochloride, 2.41-2.62 parts of histidine hydrochloride, 0.82-0.98 part of tryptophan and 0.007-0.012 part of cystine. The compound amino acid provided by the invention is accord with the growth requirements of malnourished trees, and by setting the preparation process of the compound amino acid solution, a compound amino acid solution sample with high stability can be obtained, thus avoiding great loss of the raw and auxiliary materials in the making process of the compound amino acid solution.
Owner:湖北长联杜勒制药有限公司
Lysine acetate mother liquid recycling method
InactiveCN103044196AImprove quality stabilityHigh yieldOrganic compound preparationHydroxy compound preparationAlcoholDistillation
A lysine acetate mother liquid recycling method comprises the following steps of: 1) alcohol recycle: adding sodium hydroxide and EDTA-2Na (ethylenediamine tetraacetic acid disodium salt) into a lysine acetate mother liquid till the pH value of the mother liquid is 7.0-8.0, distilling the mother liquid, detecting and recycling the distilled alcohol, and storing the distillation tail liquid for use; and 2) lysine recycle: adding drinking water into the tail liquid in the step 1 for diluting the tail liquid until the concentration reaches 7%, regulating the pH value to 3.0-4.0, selectively adsorbing lysine in the tail liquid by using resin, desorbing, decolorizing, concentrating, and refining the tail liquid to obtain the lysine acetate. By the lysine acetate mother liquid recycling method, through treatment of the lysine acetate mother liquid, qualified alcohol and lysine acetate finished products are recycled, so that the production cost is effectively reduced, resources are saved and the production efficiency is improved.
Owner:YICHANG SANXIA PHARMA
Compound amino acid injection and preparation method thereof
InactiveCN106491601AIncrease supplementPromote absorptionOrganic active ingredientsPeptide/protein ingredientsAmino acid supplementArginine
The invention relates to a compound amino acid injection and a preparation method thereof. Every 1,000 mL of the injection contains 9-12 g of isoleucine, 12.5-15 g of leucine, 9-10.5 g of lysine acetate, 4-4.5 g of methionine, 6.5-7 g of phenylalanine, 7-7.5 g of threonine, 1-1.3 g of tryptophan, 14-16 g of valine, 6.5-7.5 g of alanine, 9-10 g of arginine, 0.8-1 g of aspartic acid, 0.3-0.8 g of glutamic acid, 4.5-5 g of histidine, 4.5-5 g of praline, 1.5-1.8 g of serine, 0.4-0.6 g of tyrosine, 6.5-7 g of glycine, 0.3-0.5 g of cysteine and 0.01-0.3 g of an antioxidant. Accordingly, the amino acid supplementing and absorbing effects are improved, a user can keep vigorous, the time needed when a large amount of injection is injected is shortened, drug stimulation generated when a large amount of injection is injected is reduced, the defects of an existing amino acid injection are greatly overcome, and the advantages of being reasonable in formula, durable in effect and good in human body absorption and health care effect are achieved.
Owner:安徽富邦药业有限公司
Pharmaceutical composition containing 18 types of amino acids and preparation method thereof
ActiveCN109381460AAvoid harmEasy to prepareOrganic active ingredientsMetabolism disorderArginineThreonine
The invention discloses a pharmaceutical composition containing 18 types of amino acids and a preparation method thereof. The pharmaceutical composition comprises tyrosine, leucine, isoleucine, phenylalanine, aspartic acid, valine, threonine, proline, methionine, glutamic acid, lysine acetate, arginine, alanine, glycine, histidine, serine, tryptophan, cysteine, a pH conditioning agent, and water.The pharmaceutical composition can be free of sulfites, thereby completely eliminates the safety hazards of sulfites to the human body, and makes the clinical use safer; and the expiration period of the composition is longer.
Owner:湖北一半天制药有限公司 +1
Compound amino acid injection
InactiveCN110840911AReduce preparation timeQualified and stableMetabolism disorderPharmaceutical delivery mechanismArginineThreonine
The invention discloses a compound amino acid injection, which is prepared from, by weight according to a formula, 0.40-0.60 part of aspartic acid, 0.40-0.60 part of glutamic acid, 1.00-3.00 parts ofserine, 2.00-4.00 parts of glycine, 4.00-6.00 parts of threonine, 5.00-7.00 parts of alanine, 5.00-7.00 parts of arginine, 0.80-1.20 parts of tyrosine, 12.00-18.00 parts of valine, 8.00-12.00 parts ofmethionine, 8.00-12.00 parts of phenylalanine, 12.00-18.00 parts of isoleucine, 18.00-22.00 parts of leucine, 13.00-15.00 parts of lysine acetate, 3.00-5.0 parts of proline, 4.00-6.00 parts of histidine, 5.00-6.00 parts of tryptophan, 0.40-0.60 part of cysteine, 0.40-0.60 part of sodium bisulfite, 120.00-180.00 parts of glacial acetic acid, and 1500-1700 parts of water for injection. The preparation process provided by the invention has shorter preparation time, and is suitable for preparing a qualified and stable preparation.
Owner:天津金耀集团湖北天药药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com