Notoginsenoside pharmaceutical composition and method for preparing the same and use thereof
A technique for aescin and composition, which is applied to the pharmaceutical composition of aescin and the field of preparation thereof, can solve problems such as complicated preparation process, and achieve the effects of simple production process, reduced processing procedures, and excellent synergistic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
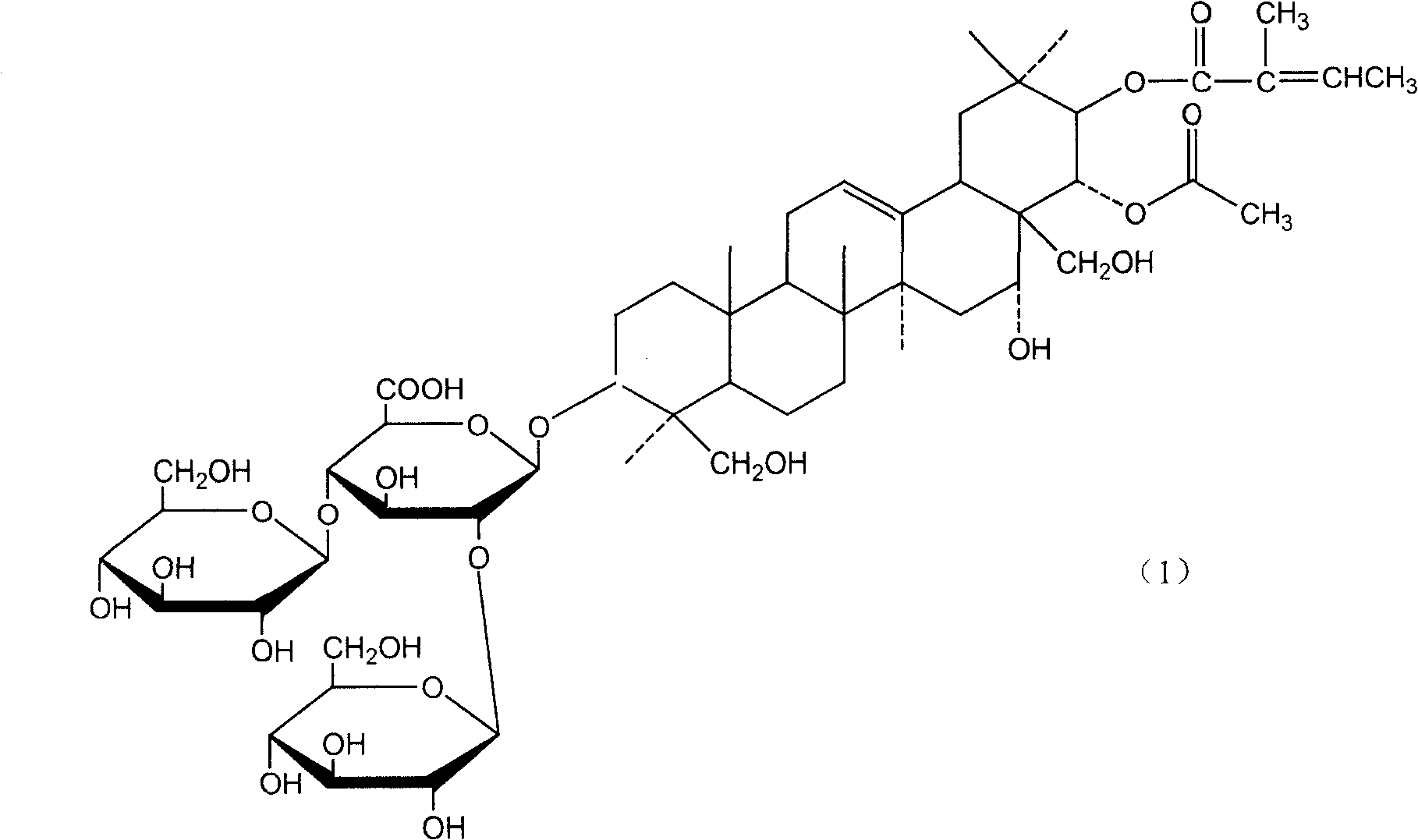
[0054] Example 1 Preparation of Potassium and Sodium Aescin Mixed Salts by Cholesterol Precipitation
[0055] Use 5 times of the amount of 5 times the amount of ethanol solution with a concentration of 50v / v%-70v / v% to stir and extract for 4 hours each time, collect and combine the extracts, recover ethanol in a vacuum to 1 / 5 of the volume of the extract, and use Determine the content of β-escin in the extract concentrate by high-performance liquid chromatography, then add cholesterol under stirring conditions with the mass ratio of β-escin and cholesterol as 1:1.2, stir for 2 hours after the addition, and place Overnight, collect the cholesterol precipitate by centrifugation the next day, wash it with water until it is colorless, dry it at 60°C, and treat it with chloroform or toluene to dissolve the cholesterol into the solvent, while the salt of aescin (sodium, potassium salt) still exists in solid form , recrystallized twice with 90%-95% ethanol to obtain white aescin sal...
Embodiment 2
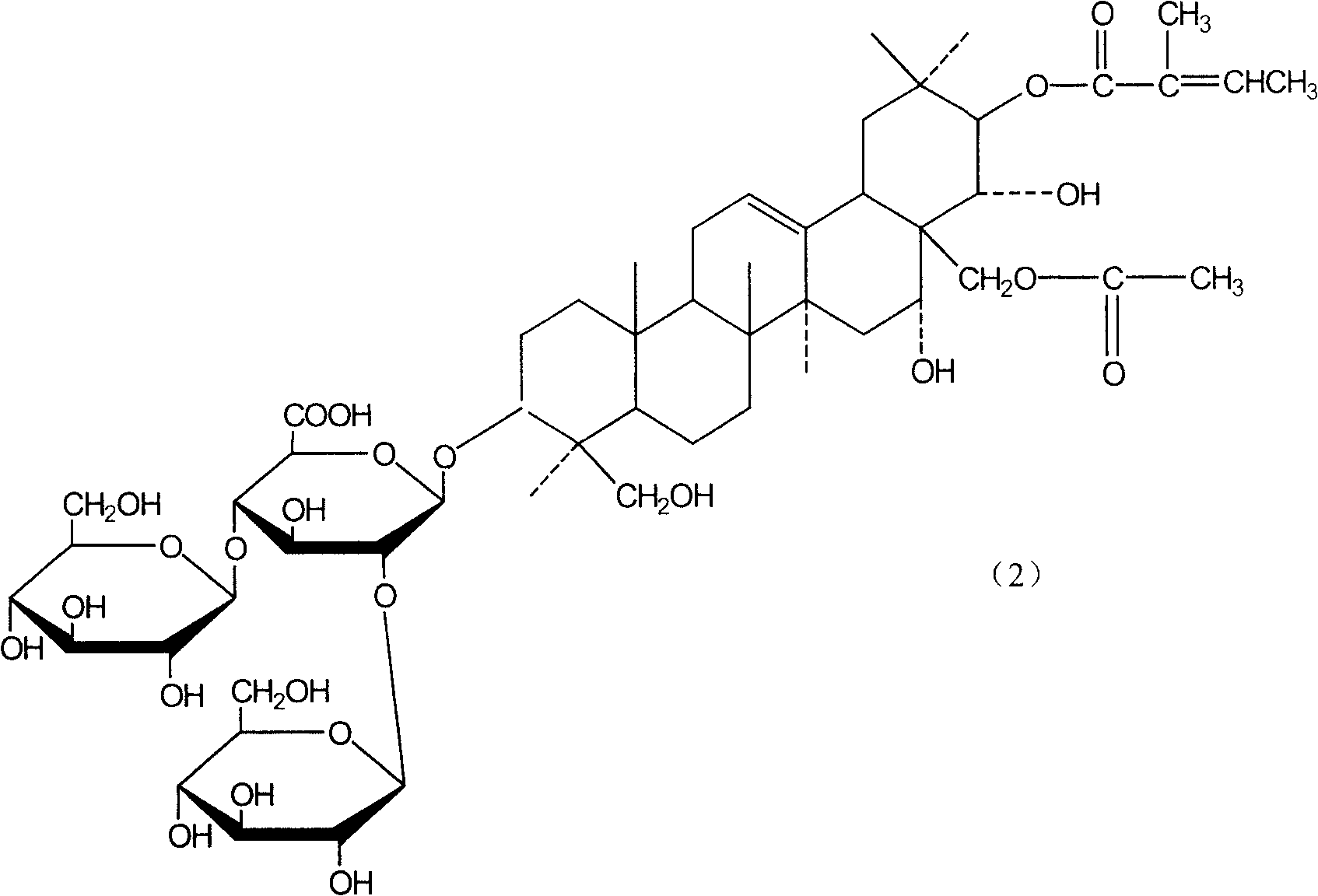
[0056] Example 2 Preparation of β-escin and isoescin
[0057] 1. Cholesterol precipitation method
[0058] Sala seed fine powder, use 5 times the amount of dilute ethanol with a concentration of 50v / v% to 70v / v% to stir and extract three times, each time for 4 hours, collect and combine the extracts, recover ethanol in a vacuum to 1 / 5 of the volume of the extract, Measure the content of β-escin in the extract concentrate by high-performance liquid chromatography, then add the cholesterol under the stirring condition with the mass ratio of β-escin and cholesterol as 1: 1.2, and stir for 2 hours after adding. Leave it overnight, and collect the cholesterol precipitate by centrifugation the next day, wash it with water until it is colorless, dry it at 60°C, and treat the precipitate with chloroform or toluene after drying to dissolve the cholesterol in the solvent, and the salt of aescin (sodium, potassium salt) still exists in solid form. After wiping off the residual solven...
Embodiment 3
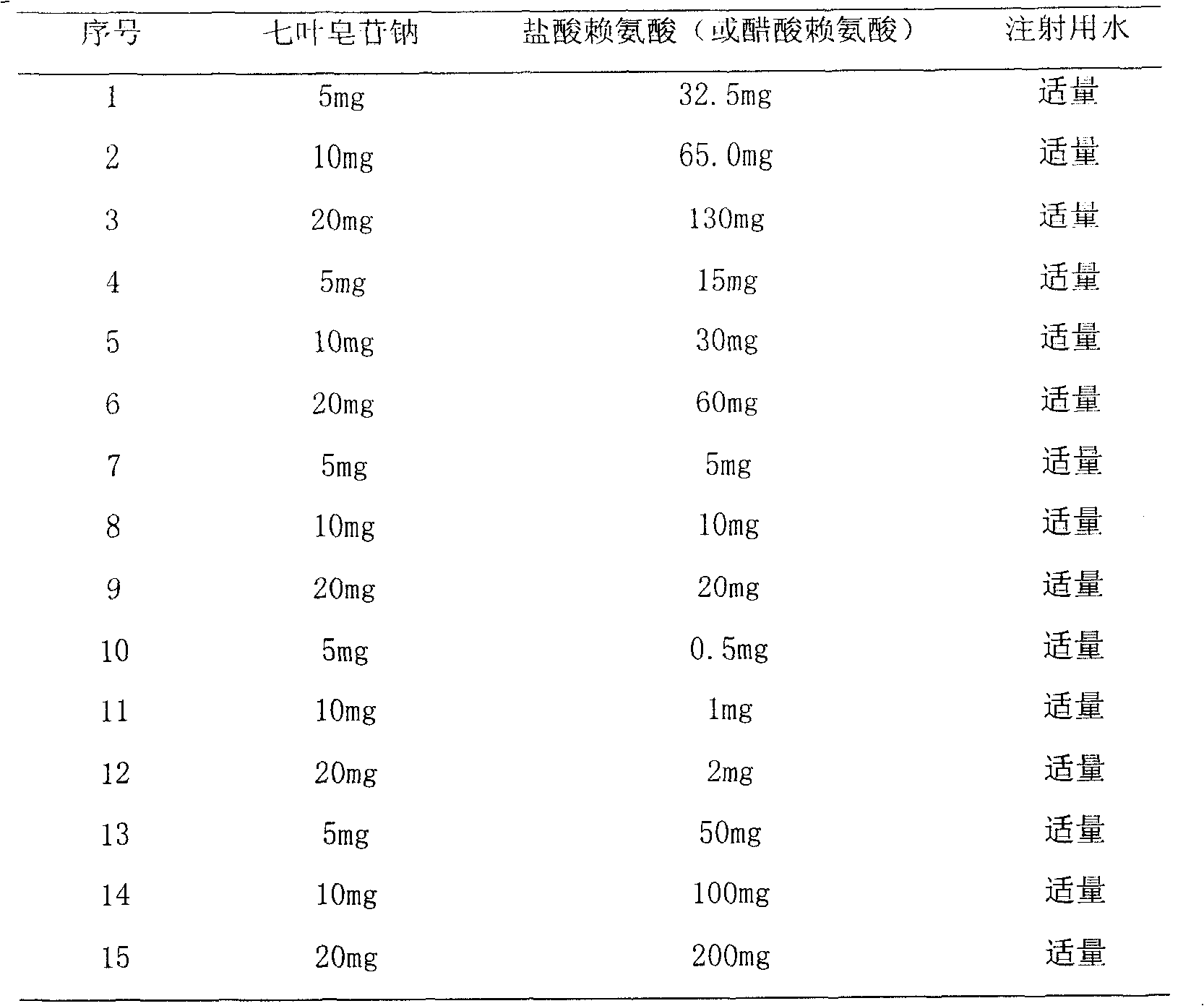
[0072] Example 3 Preparation of High Purity Sodium Aescinate
[0073] 1. Aescin salt (potassium, sodium salt) with a purity of 98.5% is dissolved in distilled water to obtain an aqueous solution with a concentration of about 2%. The aqueous solution passes through a hydrogen-type cation exchange column, and the weight ratio of aescin salt to resin is about 0.8-1.2: 1.2-0.8, to obtain a solution with a pH of about 2.0-2.8, which can be placed in a cold place or refrigerated to precipitate crystals. Redissolve the crystals in 95v / v% ethanol, add an equivalent amount of sodium hydroxide ethanol solution, place in a cold place or refrigerated to precipitate crystals, and obtain sodium aescinate crystals with a purity of 99.0%.
[0074] 2. Dissolve aescin salt (potassium, sodium salt) with a purity of 97.5% in distilled water to obtain an aqueous solution with a concentration of about 2%, add 2 mol of sulfuric acid or hydrochloric acid, adjust the pH of the solution to 1-3, and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com