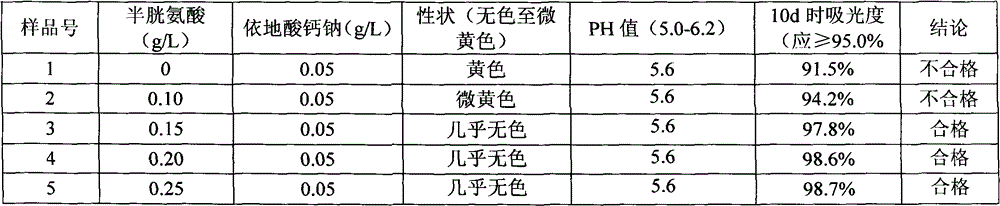
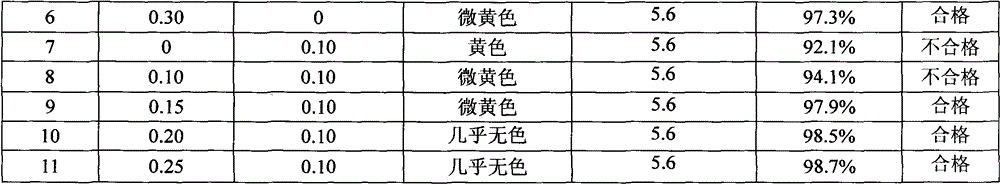
Composition containing eighteen amino acids
A composition and amino acid technology, applied in the directions of drug combination, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of affecting calcium ion level and inappropriateness, and achieve the effect of eliminating toxic side effects and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, the prescription of composition is as follows:
[0042] Aspartic acid 1.5kg, glutamic acid 2.5kg
[0043] Serine 1.9kg, Histidine 3.0kg
[0044] Glycine 3.5kg, Threonine 2.5kg
[0045] Alanine 7.2kg, Arginine 4.9kg
[0046] Tyrosine 0.2kg, cystine 0.16kg
[0047] Valine 3.2kg, Methionine 2.5kg
[0048] Tryptophan 0.85kg, Phenylalanine 3.5kg
[0049] Isoleucine 2.5kg, Leucine 3.4kg
[0050] Lysine acetate 5.5kg, proline 2.9kg
[0051] Glacial acetic acid 1300ml, cysteine 0.2kg
[0052] Calcium and sodium edetate 0.01kg, appropriate amount of water to 1000000ml.
[0053] Add about 600,000 ml of water for injection into the concentrated mixing tank, fill with nitrogen, heat to boiling and keep for 15 minutes, stop heating, and put in the above-mentioned raw and auxiliary materials in order by weight: calcium sodium edetate, cystine, tyrosine, leucine amino acid, isoleucine, valine, methionine, phenylalanine, glutamic acid, aspartic acid, lysine ace...
Embodiment 2
[0054] Embodiment 2, the prescription of composition is as follows:
[0055] Aspartic acid 2.5kg, glutamic acid 4.2kg
[0056] Serine 3.4kg, Histidine 5.0kg
[0057] Glycine 5.9kg, Threonine 4.2kg
[0058] Alanine 12.2kg, Arginine 8.4kg
[0059] Tyrosine 0.2kg, cystine 0.2kg
[0060] Valine 5.5kg, Methionine 4.2kg
[0061] Tryptophan 1.4kg, Phenylalanine 5.9kg
[0062] Isoleucine 4.2kg, Leucine 5.9kg
[0063] Lysine acetate 9.5kg, proline 5.0kg
[0064] Glacial acetic acid 2500ml, cysteine 0.2kg
[0065] Calcium and sodium edetate 0.05kg, appropriate amount of water to 1000000ml.
[0066] Add about 600,000 ml of water for injection into the concentrated mixing tank, fill with nitrogen, heat to boiling and keep for 15 minutes, stop heating, and put in the above-mentioned raw and auxiliary materials in order by weight: calcium sodium edetate, cystine, tyrosine, leucine amino acid, isoleucine, valine, methionine, phenylalanine, glutamic acid, aspartic acid, lysine acet...
Embodiment 3
[0067] Embodiment 3, the prescription of composition is as follows:
[0068] Aspartic acid 3.3kg, glutamic acid 5.7kg
[0069] Serine 4.5kg, Histidine 6.8kg
[0070] Glycine 7.9kg, Threonine 5.7kg
[0071] Alanine 16.3kg, Arginine 11.2kg
[0072] Tyrosine 0.3kg, cystine 0.2kg
[0073] Valine 7.3kg, Methionine 5.7kg
[0074] Tryptophan 1.9kg, Phenylalanine 7.9kg
[0075] Isoleucine 5.7kg, Leucine 7.9kg
[0076] Lysine acetate 12.7kg, proline 6.8kg
[0077] Glacial acetic acid 2750ml, cysteine 0.2g
[0078] Calcium and sodium edetate 0.1kg, appropriate amount of water to 1000000ml.
[0079] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com