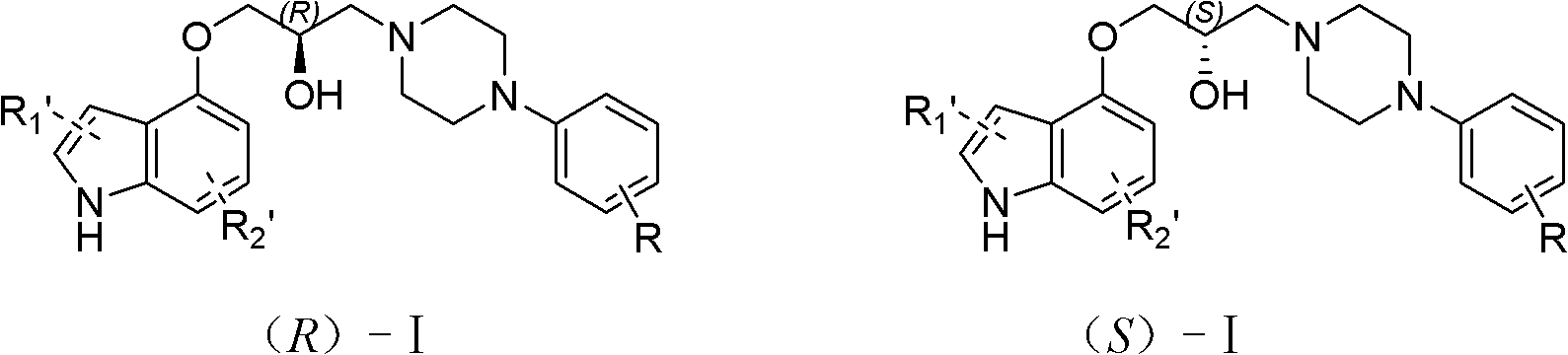
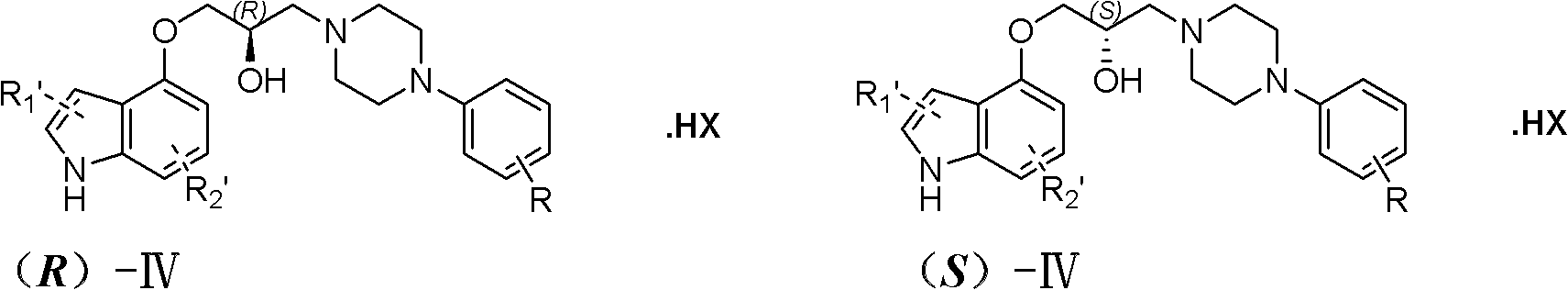1-(4-indoxyl)-3-(4-(2-methoxyphenyl)piperazidine)-2-propanol optical isomer, derivative and salt thereof and preparation and application of optical isomer
A technology of methoxyphenyl and optical isomers, applied in 1--3-piperazine)-2-propanol optical isomers, optical isomers of aryl piperazine compounds, for the preparation and treatment of hypertension and benign prostatic hyperplasia, which can solve problems such as clinical use restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of (R)-IV-1
[0042] Step 1: Preparation of R-3-(4-indoxyl)-1,2-propylene oxide
[0043] Add NaH (2.4g) and DMF into a dry four-neck flask, and stir well to make NaH a uniformly dispersed suspension. Slowly drop into the DMF solution of 6.65g (0.05mol) 4-oxindole at normal temperature, then slowly drop into the DMF solution of 13.68g (0.06mol) R-glycidyl p-toluenesulfonate after one hour, and continue the reaction at room temperature for 8 hour, water was added to remove excess NaH, the reaction was also extracted three times with ethyl acetate, the organic phases were combined, washed with water, dried over anhydrous magnesium sulfate, filtered to obtain a yellow oil, column chromatography (petroleum ether: ethyl acetate 7: 1) After further purification, 7.88 g of light yellow liquid was obtained, with a yield of 84%.
[0044] Step 2: Synthesis of o-methoxyphenylpiperazine hydrochloride
[0045] Add 8.925g (0.05mol) of dichlorodiethanolamin...
Embodiment 2
[0052] Embodiment 2: the preparation of (S)-IV-1
[0053] Step 1: Preparation of S-3-(4-indoxyl)-1,2-propylene oxide
[0054] Add NaH (2.4g) and DMF into a dry four-neck flask, and stir well to make NaH a uniformly dispersed suspension. Slowly drop into the DMF solution of 6.65g (0.05mol) 4-oxindole at normal temperature, then slowly drop into the DMF solution of 13.68g (0.06mol) S-glycidyl p-toluenesulfonate after one hour, and continue the reaction at room temperature for 8 hour, water was added to remove excess NaH, the reaction was also extracted three times with ethyl acetate, the organic phases were combined, washed with water, dried over anhydrous magnesium sulfate, filtered to obtain a yellow oil, column chromatography (petroleum ether: ethyl acetate 7: 1) After further purification, 7.01 g of light yellow liquid was obtained, with a yield of 74.6%.
[0055] Step 2: Synthesis of S-1-(2-methoxyphenyl)-4-[3-(4-indyloxy)-2-hydroxypropyl]piperazine
[0056] 3.84g (0.02m...
Embodiment 3
[0059] Embodiment 3: Preparation of (R)-IV-2
[0060] Step 1: Preparation of R-3-(4-indoxyl)-1,2-propylene oxide
[0061] Add NaH (2.4g) and DMF into a dry four-neck flask, and stir well to make NaH a uniformly dispersed suspension. Slowly drop into the DMF solution of 6.65g (0.05mol) 4-oxindole at normal temperature, then slowly drop into the DMF solution of 13.68g (0.06mol) R-glycidyl p-toluenesulfonate after one hour, and continue the reaction at room temperature for 8 hour, water was added to remove excess NaH, the reaction was also extracted three times with ethyl acetate, the organic phases were combined, washed with water, dried over anhydrous magnesium sulfate, filtered to obtain a yellow oil, column chromatography (petroleum ether: ethyl acetate 7: 1) After further purification, 8.44 g of light yellow liquid was obtained, with a yield of 90%.
[0062] Step 2: Synthesis of o-chlorophenylpiperazine hydrochloride
[0063]Add 8.925g (0.05mol) of dichlorodiethanolamine ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com