Conjugated proteins
A protein, conjugation technology, applied in the field of modified proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
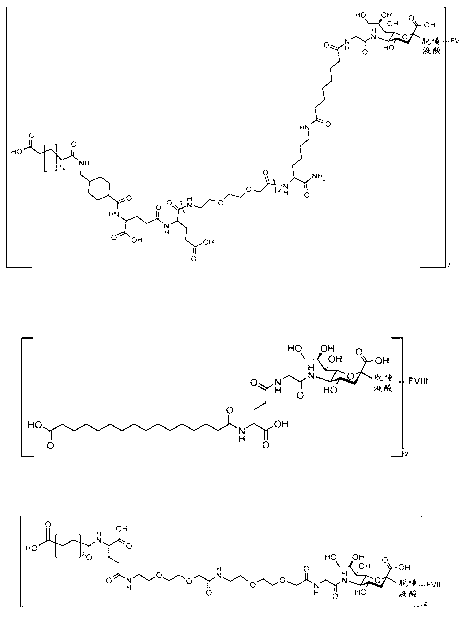
[0204] Production of recombinant B-domain truncated O-glycosylated factor VIII
[0205] Cell Lines and Culture Methods
[0206] Mammalian expression plasmids were constructed using Factor VIII cDNA. The plasmid encodes Factor VIII with a B domain deletion, a Factor VIII heavy chain comprising amino acids 1-740 of full-length human Factor VIII, a Factor VIII light chain comprising amino acids 1649-2332 of full-length human Factor VIII (this molecule may be referred to herein as for "N8", see Thim et al., Haemophilia (2010) 16 , 349). The heavy and light chain sequences are separated by a 21 amino acid linker (SFSQNSRHPSQNPPVLKRHQR- SEQ ID NO 4 ) linkage, the linker comprising the sequence of amino acids 741-750 and 1638-1648 of full-length human Factor VIII. Chinese hamster ovary (CHO) cells were transfected with the plasmid, selected with the dihydrofolate reductase system, and finally produced clonal suspension producer cells cultured in animal component-free medium....
Embodiment 2
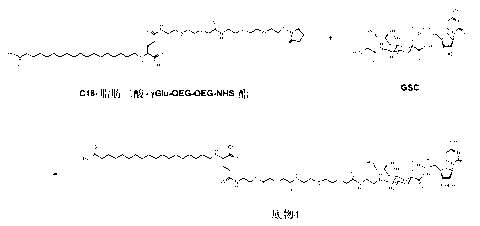
[0217] Conjugation of C-16 fatty diacid γ-Glu NHS ester to GSC to obtain sialyltransferase substrate 1
[0218]
[0219] GSC (glycylsialic acid CMP ester) (7.5 mg, 11 μmol) was dissolved in a mixture of TRIS buffer (100 mM, pH 8.4, 50 μl) and acetonitrile 50 μl. A two-phase system is obtained. C-16 fatty diacid γ-Glu NHS ester (7 mg; see WO 2005 / 012347 A2 Example 5 for the preparation of the analogue C-18 compound) was dissolved in THF (50 μl) and TRIS-buffer was added (50 μl), a clear solution was obtained. This solution was added to the solution of GSC. A clear solution was obtained. After about 4 hours of reaction time, the samples were concentrated in vacuo to remove most of the organic solvent and then frozen at -20°C. product( 1 ) identified by HPLC and LC-MS.
[0220] Purified by RP-HPLC using a neutral buffer system and a C4 Jupiter 10x250 cm column. Flow rate 5 ml / min, gradient 2% B buffer / min. collection of products 1 late elution fraction. Fractions w...
Embodiment 3
[0222] Example 3. Preparation of albumin binder NHS ester 2
[0223]
[0224] Rink-amide resin (Novabiochem, 0.4 g, 0.25 mmol) was used for synthesis on a CEM Liberty microwave peptide synthesizer. A standard Fmoc chemistry scheme was employed and the following amino acids used were used in this order (all solutions had 7 equivalents of amino acids in NMP containing 0.3 M HOAt):
[0225] 1. Fmoc-Lys(Mtt)-OH: 1.12 g / 6 ml
[0226] 2. Fmoc-OEG-OH: 1.39 g / 12 ml (2 couplings)
[0227] 3. Fmoc-Glu-OtBu: 0.77 g / 6 ml
[0228] 4. Fmoc-Thx-OH: 0.68 g / 6 ml
[0229] 5. Mono-tert-butyl C-16 diacid (see Example 4 of WO 2005 / 012347 A2): 0.62 g / 6 ml
[0230] All couplings were performed by addition of DIC 7 eq.
[0231] After these couplings, the resin was removed from the Liberty synthesizer and washed, drained, and treated with 5 ml of hexafluoroisopropanol for 10 minutes. The resin was then washed with DCM and drained. Then, the hexafluoroisopropanol treatment and DCM wash were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com