Method for purifying solar grade polysilicon
A solar-grade, polysilicon technology, applied in chemical instruments and methods, silicon compounds, inorganic chemistry, etc., can solve the problems of complex process, high cost, and impermanent purity, and achieve low equipment investment, low cost, and process operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
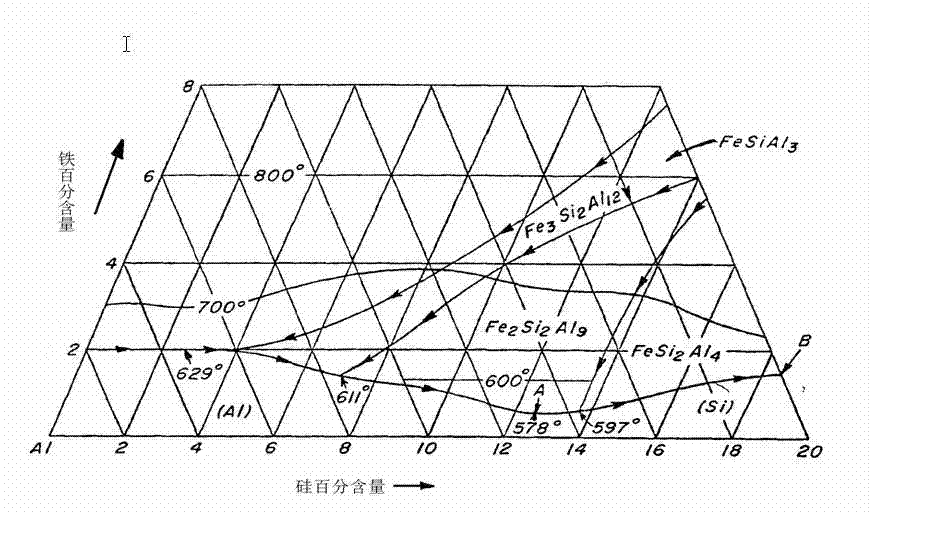
[0019] Example 1 Add aluminum to silicon containing 99.0% by weight of silicon, the weight ratio of aluminum is 99.5%-99.9%, the content of iron in the mixture is not more than 0.8%, and the weight ratio of raw material silicon to aluminum is: 20-30 : 80-70, at the same time add titanium with a weight ratio not exceeding 0.2% of the total weight, and heat it. When the silicon is completely melted in the aluminum, let it stand for 1-4 hours and separate the precipitated boron, and then place it at the bottom of the melt Introduce chlorine gas, blow from bottom to top for 10-40 minutes, phosphorus will concentrate on the surface of the melt, remove phosphorus with traditional methods, start cooling at a rate of 2 degrees per minute, and cool until aluminum is discharged from the crystallization bed out, keep the temperature of the bed at this temperature, and continue to lower the temperature, which is conducive to the outflow of aluminum, and the aluminum remaining on the crysta...
Embodiment 2
[0021] Example 2, adding aluminum with a weight ratio of 99.9% purity to silicon with a weight ratio of 99.0%, silicon 25% (weight ratio), aluminum 74% (weight ratio), and the rest are impurities. Titanium that exceeds 0.2% of the total weight is heated to 850 degrees, and the silicon is melted in the aluminum. After the silicon is melted, the precipitated boron is separated after standing for 1-4 hours. Chlorine gas is introduced at the bottom of the melt and blown from bottom to top Gas for 10-40 minutes, phosphorus will concentrate on the surface of the melt, remove phosphorus with traditional methods, start to cool at a rate of 2 degrees per minute, and cool to 585 degrees, aluminum is discharged from the crystallization bed, and the bed is The temperature is maintained at 585 degrees, which facilitates the outflow of aluminum, leaving about 49% of the aluminum on the crystallization bed, allowing the remaining material to partially remelt, and then cooling, reducing the am...
Embodiment 3
[0022] Embodiment 3: Operation is the same as example 2 before the discharge of the molten phase, and the crystallization bed is treated with a gas flame after the discharge of the molten phase, so that the remaining material part is re-melted, and the amount of residual aluminum is reduced by 42% on average.
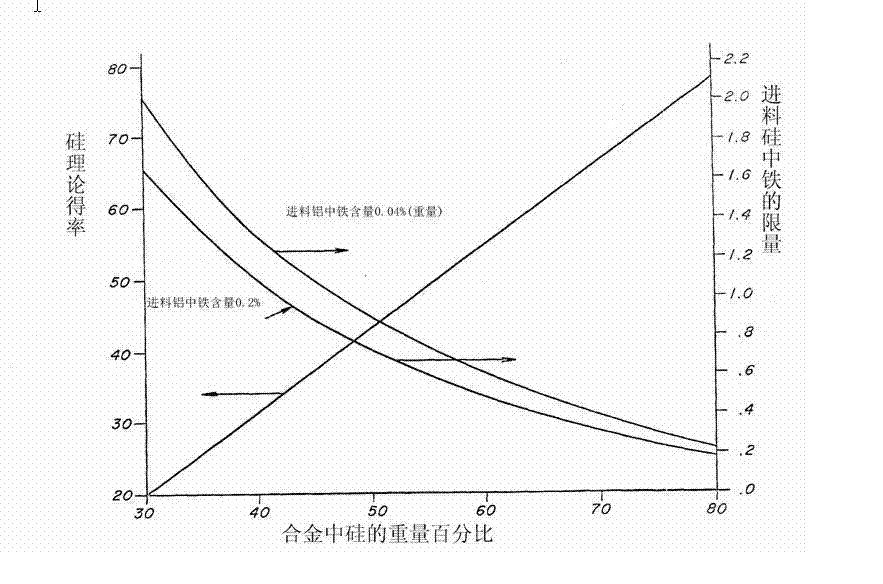
[0023] When the silicon is 99.0% pure by weight, the added aluminum can be commercial grade, ie 99.5% pure by weight. Of course, it is better if the purity of aluminum reaches 99.9%, because there are fewer impurities brought in. Moreover, silicon containing a large amount of impurities has no negative impact on the quality of high-purity silicon purified by this method, but in order to obtain high-purity silicon in an economical manner, the amount of other substances such as impurities should be controlled for silicon a certain range. When using aluminum as a flux, the iron content in the molten alloy must be controlled at such a level that during fractional crystalli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com