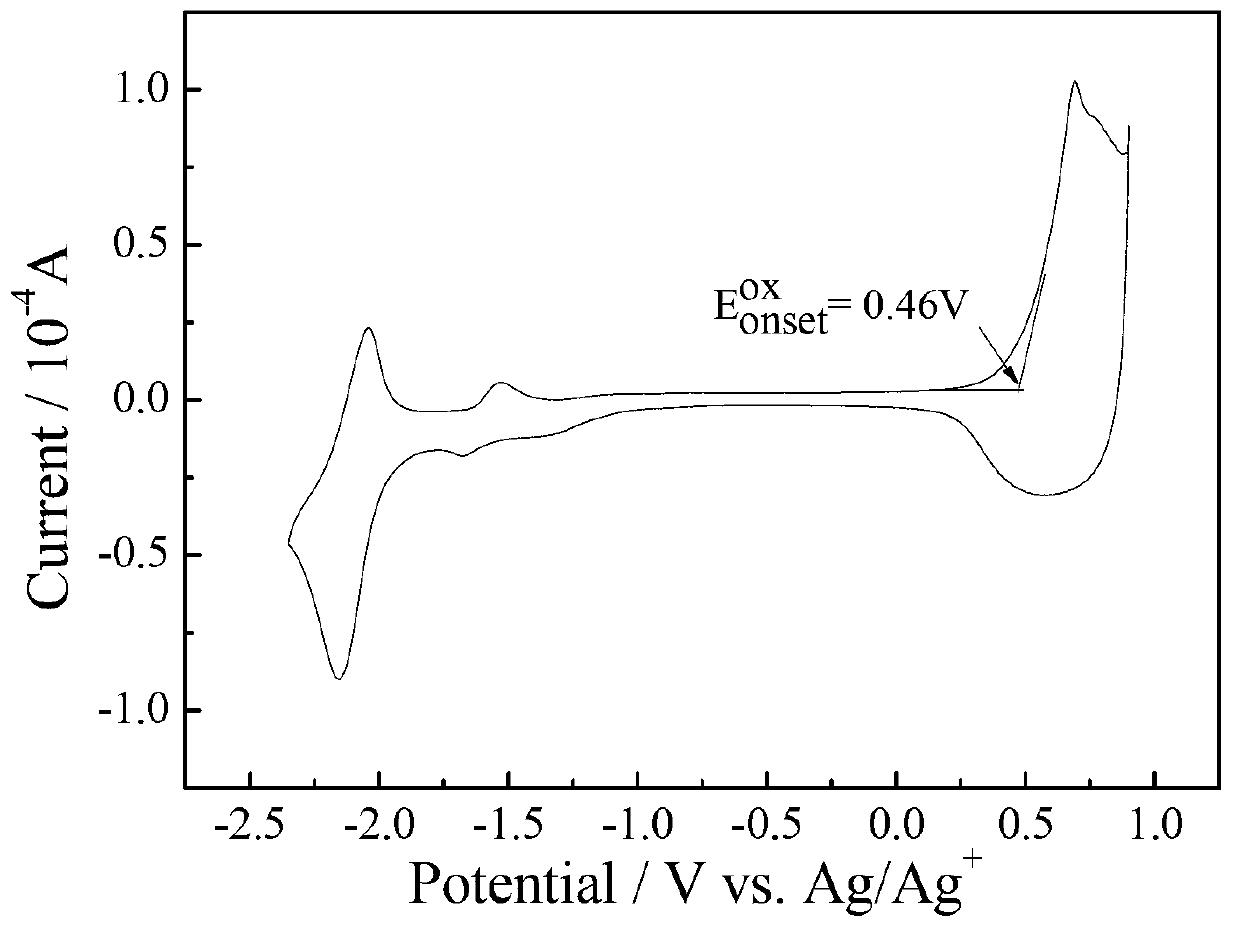
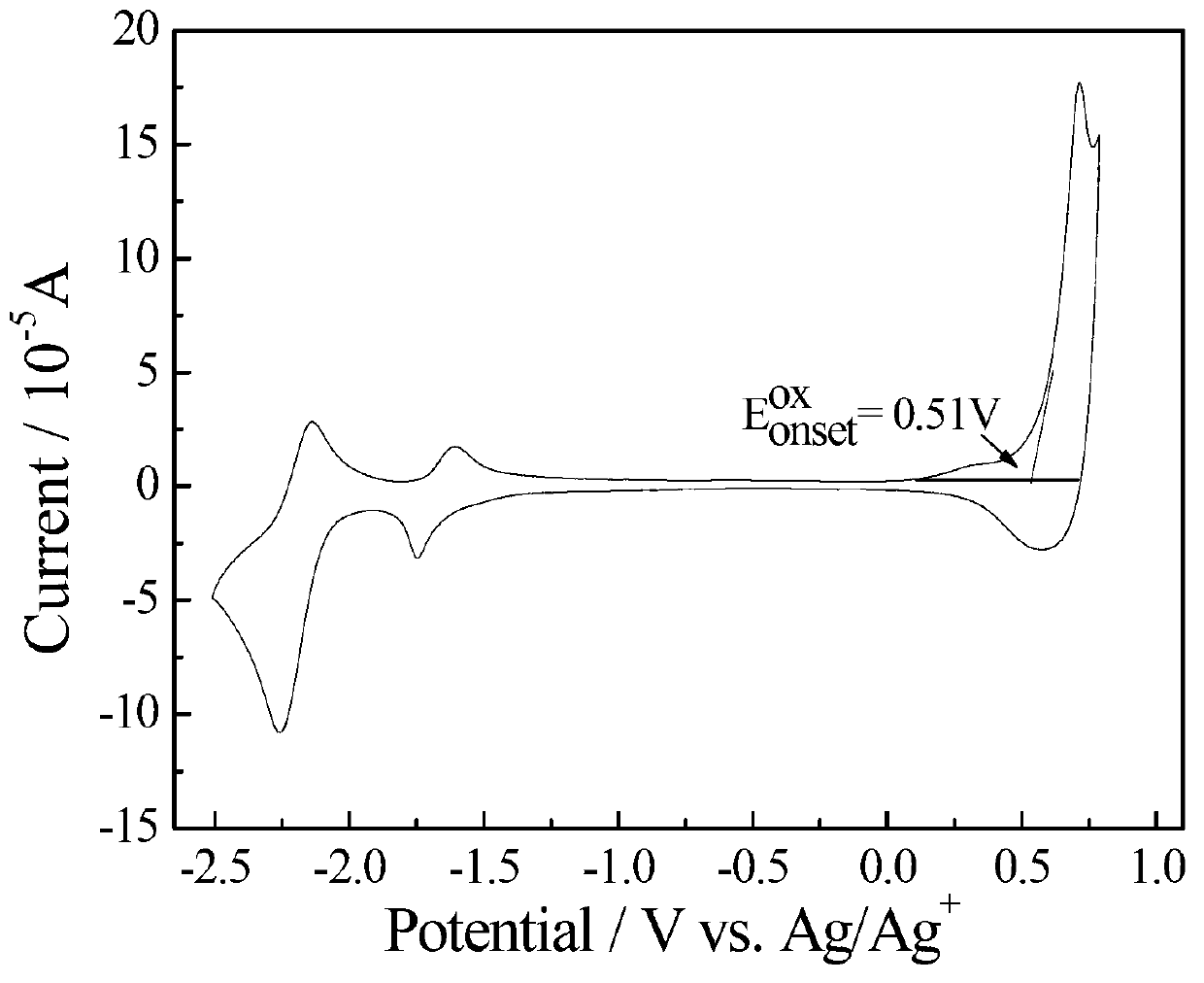
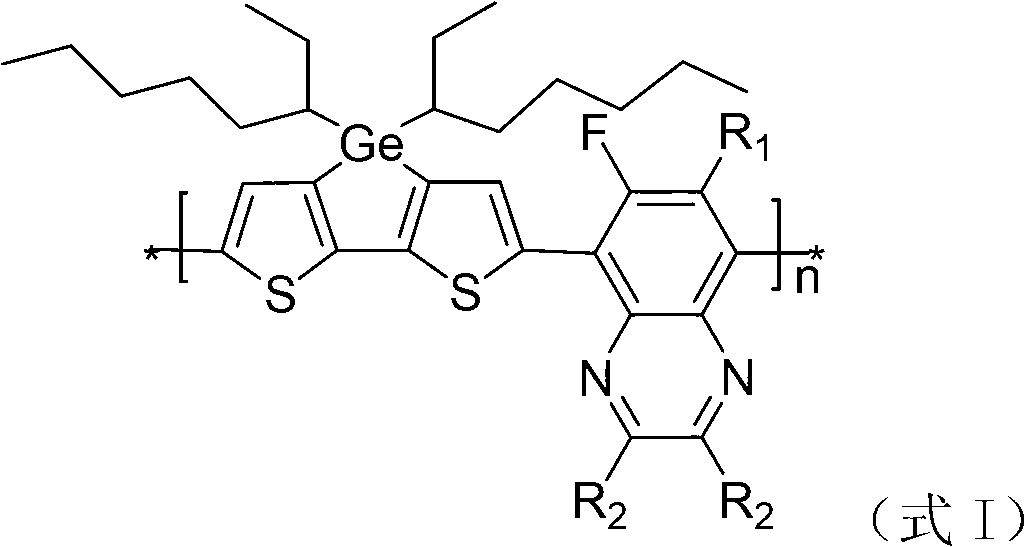
Dithiophene ring-fused germanium pentadiene-fluoroquinoxaline conjugated polymer
A conjugated polymer, bisthieno technology, applied in the field of bisthienocyclodermopentadiene-fluoroquinoxaline conjugated polymers, to achieve the effect of increasing the open circuit voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Synthesis of 6-fluoro-5,8-dibromo-2,3-bis(3-octyloxyphenyl)quinoxaline as an electrolytic unit
[0036] (1) Preparation of 4-fluoro-3,6-dibromo-1,2-phenylenediamine (compound 2)
[0037] Follow the reaction equation shown below:
[0038]
[0039] 5-Fluoro-4,7-dibromo-2,1,3-benzothiadiazole (5 g, 0.016 mol) was dissolved in 150 ml of absolute ethanol, and NaBH was added in batches at 0 °C 4 (11.1g, 0.29mol), and then reacted at room temperature for 20h. After the reaction, spin off ethanol, add 160ml of water, extract with ethyl acetate, wash the organic phase with saturated brine, and finally anhydrous MgSO 4 dry. The organic solvent was removed by concentration, and the obtained crude product was purified by a silica gel column using n-hexane / ethyl acetate (25:1, v / v) as the eluent to obtain compound 2, m=3.5g, and the yield was 78%.
[0040] (2) Preparation of 1,2-bis(3-octyloxyphenyl)ethanedione (compound 5)
[0041] Follow the reaction equation sh...
Embodiment 2
[0049] Example 2: Synthesis of 6-fluoro-5,8-dibromo-2,3-bis(4-octyloxyphenyl)quinoxaline (compound 8)
[0050] Follow the reaction equation shown below:
[0051]
[0052] Using 4-fluoro-3,6-dibromo-1,2-phenylenediamine and 1,2-bis(4-octyloxyphenyl)ethanedione as raw materials, completely follow the method for the synthesis of compound 6 to obtain the compound 8. The yield is 82%.
Embodiment 3
[0054] Synthesis of the electroporous unit 6,7-difluoro-5,8-dibromo-2,3-bis(3-octyloxyphenyl)quinoxaline (compound 11)
[0055] (1) Preparation of 4,5-difluoro-3,6-dibromo-1,2-phenylenediamine (compound 10)
[0056] Follow the reaction equation shown below:
[0057]
[0058] 5,6-difluoro-4,7-dibromo-2,1,3-benzothiadiazole (10 g, 0.031 mol) was dissolved in 300 ml of absolute ethanol, and NaBH was added in batches at 0 °C 4 (22.2g, 0.59mol), then reacted at room temperature for 5h. After the reaction, concentrate to remove ethanol, add 200ml of water, extract with ethyl acetate, wash the organic phase with saturated brine, and finally anhydrous MgSO 4 dry. The obtained crude product was concentrated and purified by silica gel column chromatography, and the eluent was n-hexane / ethyl acetate (20:1, v / v) to obtain 4,5-difluoro-3,6-dibromo-1 , 2-phenylenediamine 6.1g, yield 65%.
[0059] (2) Preparation of 6,7-difluoro-5,8-dibromo-2,3-bis(3-octyloxyphenyl)quinoxaline (compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com