Medicinal composition of agomelatine-containing enteric-coated tablets
A technology of composition and enteric-coated tablet, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
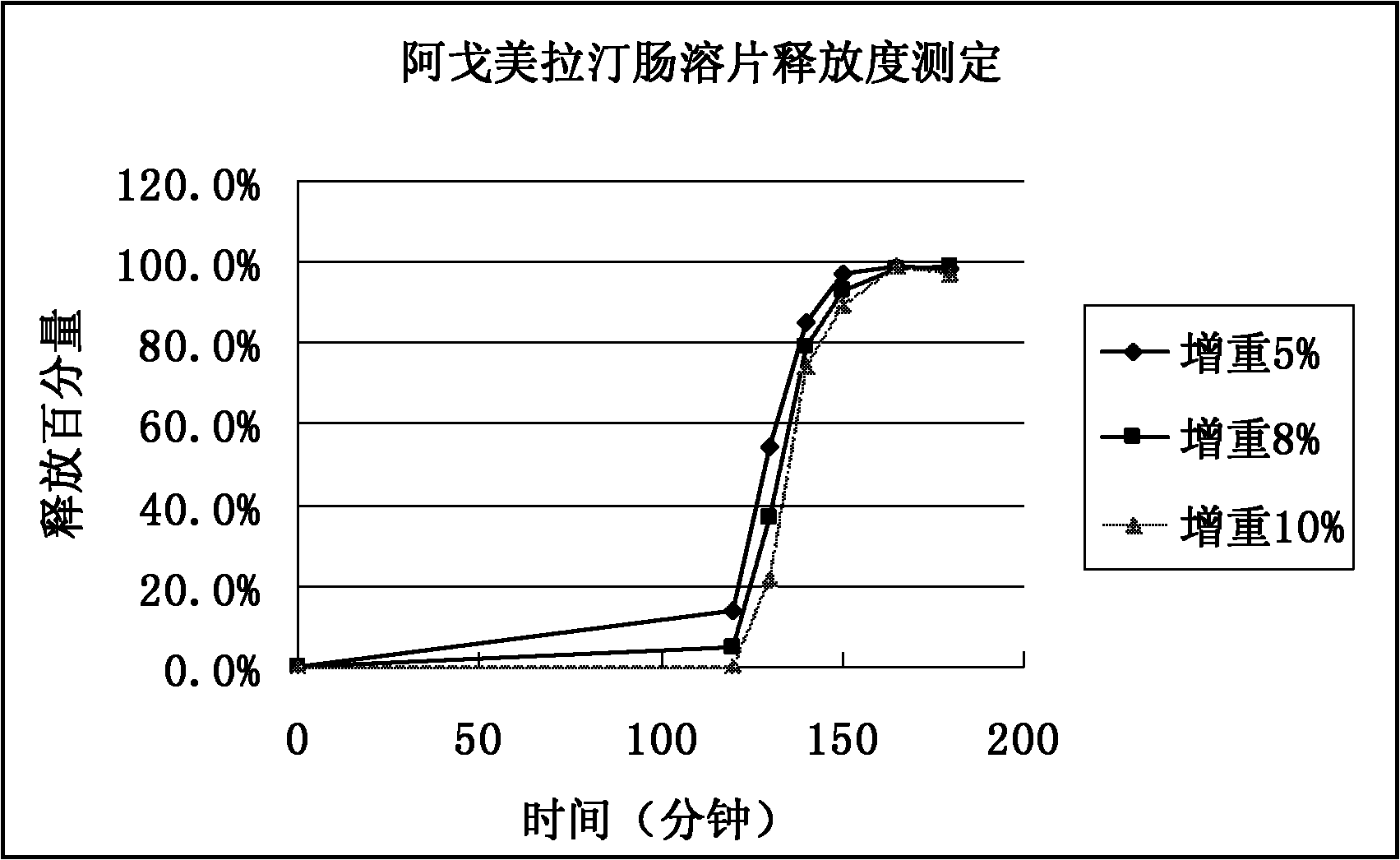
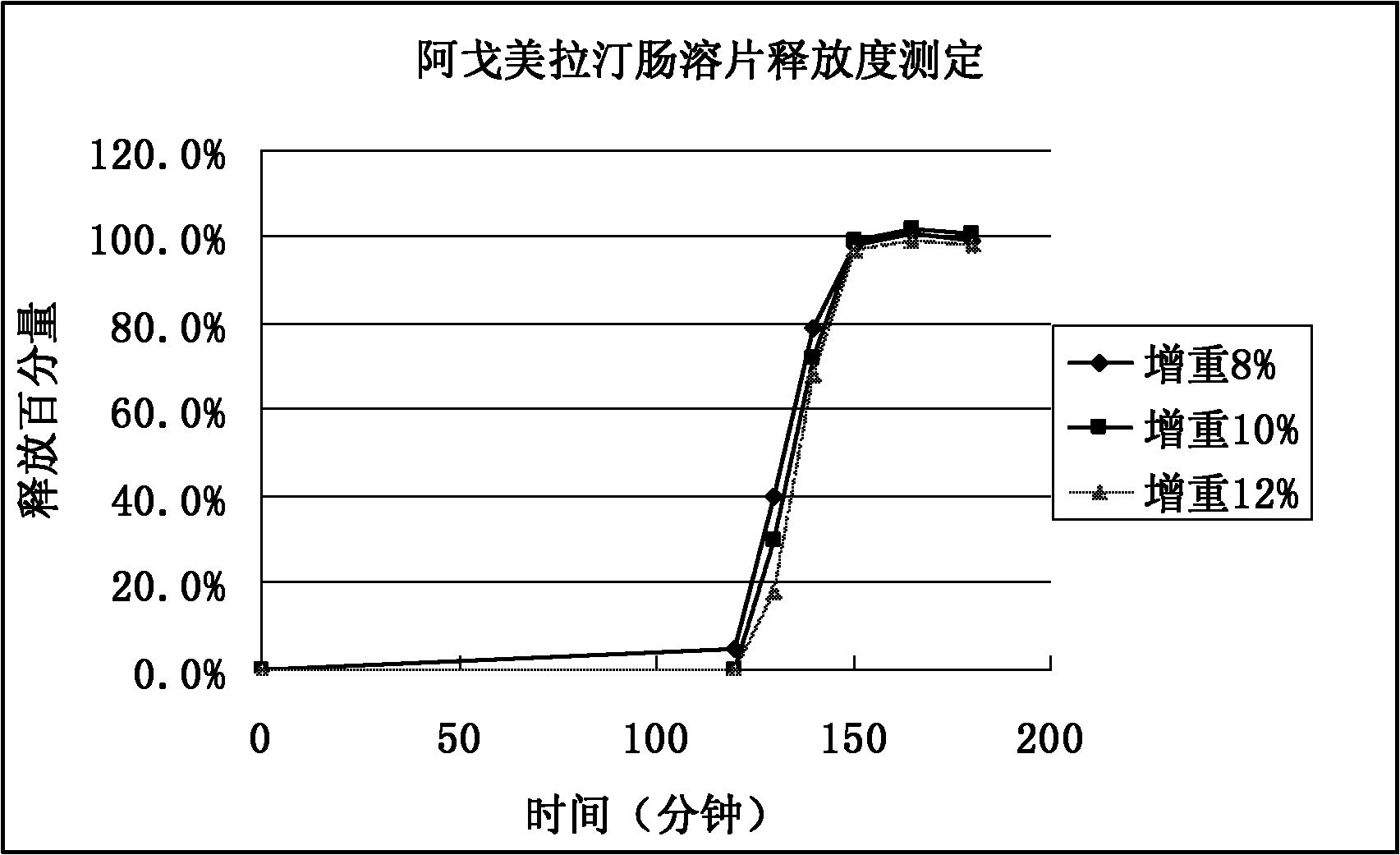
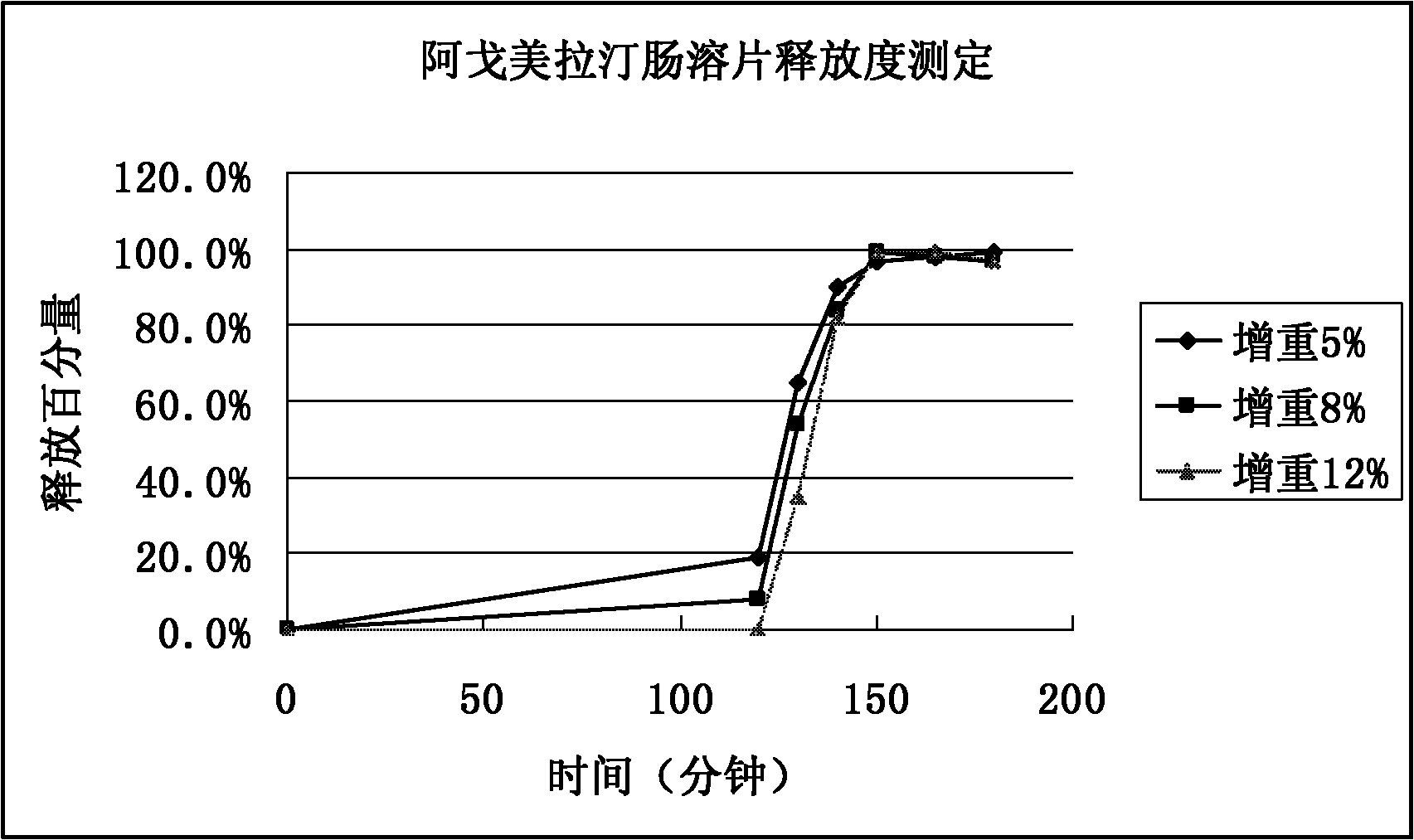
Image
Examples
Embodiment 1
[0034] Enteric-coated tablet containing 2 mg agomelatine
[0035] The first five of the following substances were blended together, wet granulated, dried and sized. The granules are then mixed with magnesium stearate and compressed in batches of 100 mg tablets each using a rotary tablet press.
[0036]
[0037] Isolation gown
[0038] Povidone 30g
[0039] water 500g
[0040] Process: a, the povidone of prescription quantity is dissolved in the water of prescription quantity,
[0041] b. Preheat the plain tablets in the coating pan so that the temperature of the tablet bed is 40°C.
[0042] c. Coating is completed at a speed of 3ml / min.
[0043] Enteric coating layer
[0044]
[0045] Process: a. Disperse the Eudragit S of the prescribed amount in 30g of water,
[0046] B, sodium hydroxide solution (1mol / l) is dripped under stirring condition to complete, and continue to stir for 30 minutes,
[0047] c, add triethyl citrate and talcum powder of prescription quant...
Embodiment 2
[0051] Enteric-coated tablet containing 3 mg agomelatine
[0052] The first five of the following substances were blended together, wet granulated, dried and sized. The granules were then mixed with silicon dioxide and compressed in batches of 150 mg tablets each using a rotary tablet press.
[0053]
[0054] Isolation gown
[0055] Hypromellose (50cp) 30g
[0056] water 1000g
[0057] Process: a, the hypromellose of prescription quantity is dissolved in the water of prescription quantity,
[0058] b. Preheat the plain tablets in the coating pan so that the temperature of the tablet bed is 40°C.
[0059] c. Coating is completed at a speed of 3ml / min.
[0060] Enteric coating layer
[0061]
[0062] Process: a. Mix the components in the prescription according to the prescription amount for later use,
[0063] b. Preheat the above-mentioned coated tablets in a coating pan so that the tablet bed temperature is 40°C,
[0064] c. Coating at a speed of 4ml / min, samplin...
Embodiment 3
[0066] Enteric-coated tablet containing 5 mg agomelatine
[0067] The raw and auxiliary materials are mixed, and a rotary tablet press is used for direct tablet compression, and a batch of tablets with a quality of 200 mg per tablet is pressed.
[0068]
[0069] Isolation gown
[0070] Hypromellose (50cp) 50g
[0071] Ethanol 600g
[0072] water 1400g
[0073] Process: a, the hypromellose of prescription quantity is dissolved in the 30% ethanol of prescription quantity,
[0074] b. Preheat the plain tablets in the coating pan so that the temperature of the tablet bed is 40°C.
[0075] c. Coating is completed at a speed of 3ml / min.
[0076] Enteric coating layer
[0077]
[0078] Process: a. Dissolve Eudragit L100 in ethanol,
[0079] b, add triethyl citrate and talcum powder of prescription quantity, make coating solution for subsequent use,
[0080] d. Preheat the above-mentioned coated tablets in a coating pan so that the tablet bed temperature is 40°C
[0081...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com