Tetravalent dengue virus subunit vaccine, its preparation method and application thereof
A dengue virus, vaccine technology, applied in antiviral agents, viral antigen components, resistance to vector-borne diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
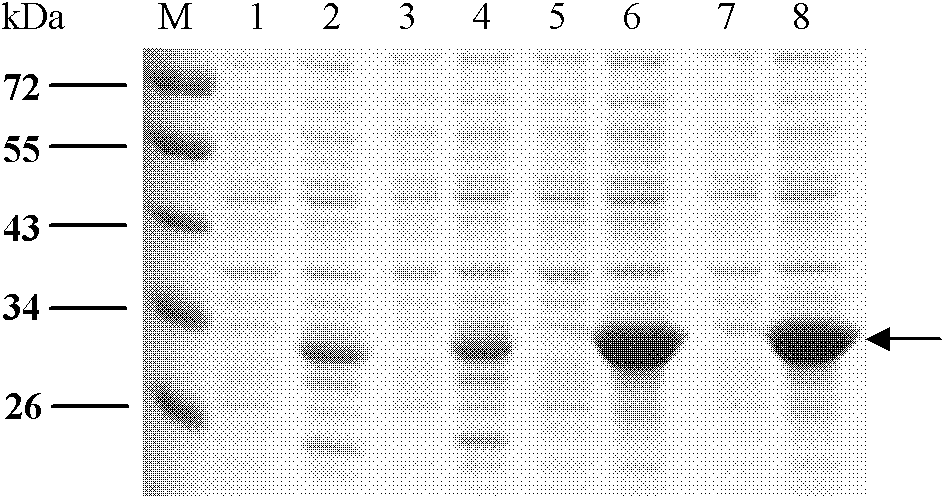
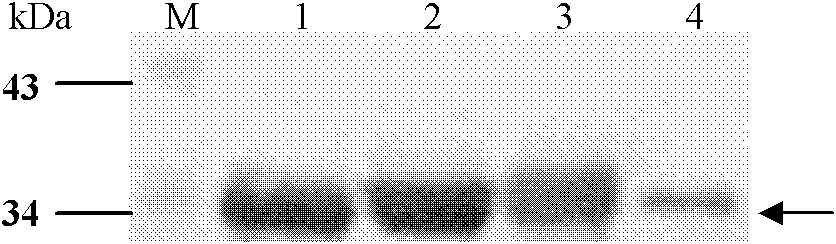
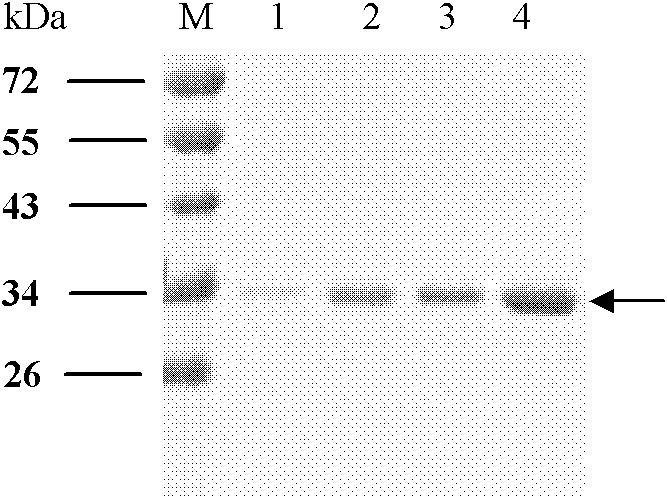
[0049] Embodiment 1, the preparation of four kinds of albumen
[0050] 1. E structural protein domain III protein of dengue virus DENV1
[0051] 1. Gene
[0052] Use dengue type 1 virus GZ / 80 strain RNA (the virus sequence has the 1st-10735th nucleotide sequence of GENBANK number AF350498) as a template, or use the artificially synthesized gene shown in SEQ ID NO: 5 as Template, PCR amplification was carried out with the primers shown in DIII1-F / DIII1-R in Table 1.
[0053] The PCR cycle conditions were 94°C for 2min; 94°C for 30sec, 55°C for 30sec, and 72°C for 1min, a total of 35 cycles; 72°C for 7min.
[0054] As a result, an amplified fragment with a length of about 300 bp was obtained, which was consistent with the gene length of the DIII region of E protein.
[0055] 2. Recombinant expression vector
[0056] The PCR amplified product obtained in step 1 was digested with EcoR V and Sal I at 37°C for 3 hours, and the purified fragment was recovered and ligated with the...
Embodiment 2
[0084] Embodiment 2, animal immunization and immune effect evaluation
[0085]Four-week-old Balb / C female mice were selected for immunization. Mice were divided into 2 groups (DIII mixed group and Trx group), 6 mice / group. Mix the purified DIII proteins of the 4 serotypes of dengue virus in a mass ratio of 1:1:1:1 (the immunization dose is 25 μg for the 4 proteins respectively) or dilute 25 μg of thioephrin (Trx) in 100 μl of PBS , and then mixed with Freund's complete adjuvant at a volume ratio of 1:1, fully emulsified and injected subcutaneously at multiple points for primary immunization, and each mouse was injected with 200 μl. Booster immunization once every 2 weeks, a total of 3 booster immunizations. The dosage and immunization route of each booster immunization were the same as those of the initial immunization, and the protein and Freund's incomplete adjuvant were mixed at a volume ratio of 1:1 during the booster immunization. Before the first immunization and two ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com