Method for preparing almotriptan malate
A technology of almotriptan and malic acid, applied in the field of medicinal chemical synthesis, can solve the problems of insecurity, increased production cost, inconvenience and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Synthesis of Almotriptan Malate
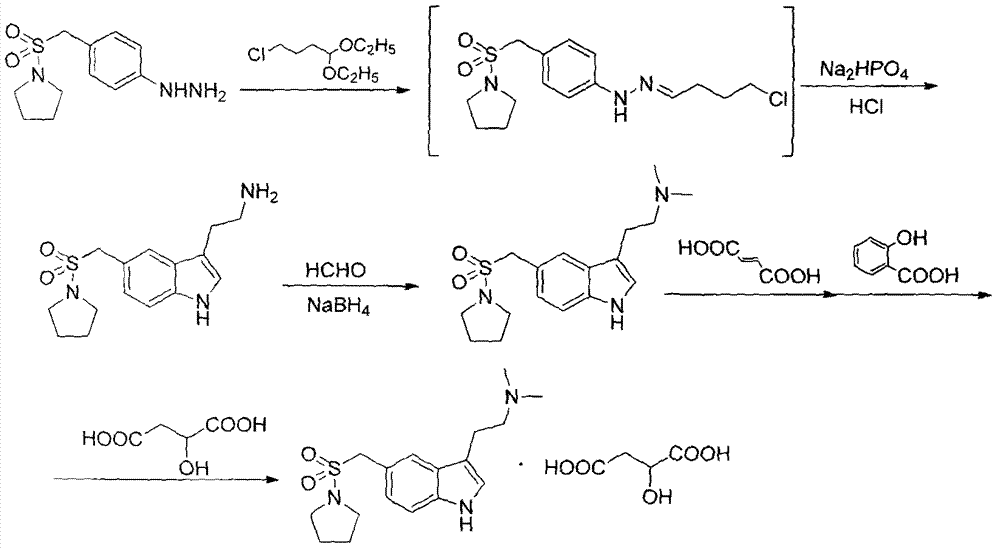
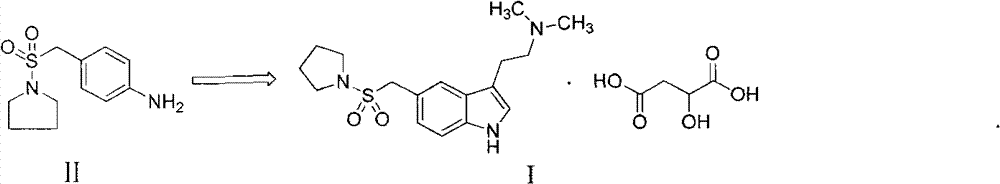
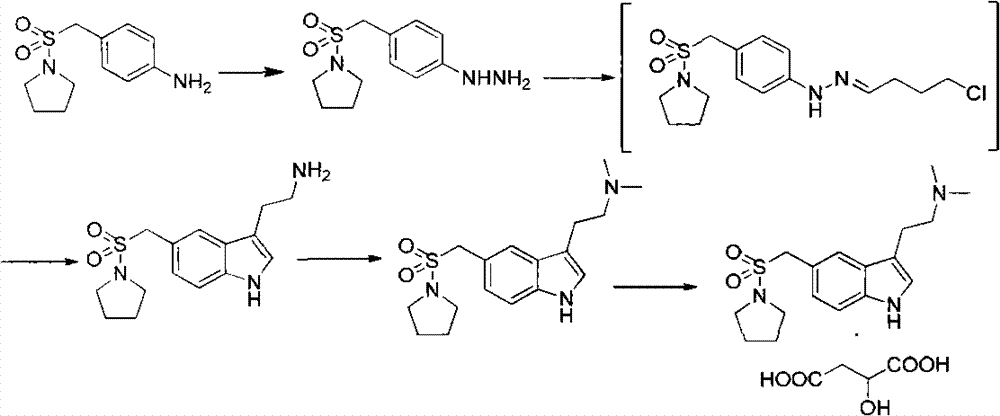
[0024] 1.1 Preparation of 3-[2-(dimethylamino)ethyl]-5-(1-pyrrolidinylsulfonylmethyl)-1H-indole
[0025] Reaction equation:
[0026]
[0027] Steps:
[0028] 19.3L of water, 380ml of concentrated hydrochloric acid and 1556.0g of 4-chlorobutyraldehyde diethyl acetal were added to the 50L reaction kettle, stirred at room temperature for 1 hour, and used for later use.
[0029] Under nitrogen protection, put 21.4L of methanol, 2000g of 4-(1-pyrrolidinylsulfonylmethyl)phenylhydrazine, 4.6L of water and 700ml of concentrated HCl into the stirred 100L reactor, dropwise at 20±5°C Add the 4-chlorobutyraldehyde diethanol acetal solution prepared above. During the dropwise addition, a yellow solid was precipitated. After the dropwise addition was completed, the mixture was stirred at 0-5° C. for 2 hours. TLC monitoring of raw materials basically completed the reaction. Centrifuge and filter to dryness to obtain a yellow solid. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com