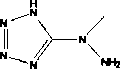
5-(1'-methyl)hydrazinotetrazolium and its metal salt derivative
A technology of hydrazine tetrazole and metal salt, applied in the field of new chemical materials and preparation thereof, can solve the problems of dangerous operation, unstable structure, difficult synthesis and the like, and achieve the effects of easy operation, high product purity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, 5-(1 ' -Methyl)hydrazinotetrazole
[0026] Put 4.00 g (38 mmol) of cyanogen bromide in the reaction flask, add 50 mL of acetonitrile, and stir to dissolve at 0 °C. Add 10.00 g (154 mmol) sodium azide solid, stir the reaction at 0 °C for 4 h, and filter. The filtrate was added dropwise to a 30 mL acetonitrile solution containing methylhydrazine (0.92 g, 20 mmol), stirred for 4 h, and filtered. The precipitate was dried in vacuo at 80 °C to obtain a white solid, namely 5-(1 ' -methyl)hydrazinotetrazole. Yield: 73%. Take a small amount of white solid and dissolve it in a small amount of acetonitrile, slowly cool and let it stand, and obtain colorless transparent flaky crystals after 1 day, which is 5-(1 ' -Methyl)hydrazinotetrazole crystals.
[0027] The reaction formula is: BrCN+NaN 3 +CH 3 NHNH 2 à [CH 3 N(NH 2 ) CN 4 H]
Embodiment 2
[0028] Embodiment 2, 5-(1 ' -Methyl)hydrazinotetrazole
[0029] Put 1.06 g (10 mmol) cyanogen bromide in the reaction flask, add 0.65 g (10 mmol) sodium azide solid, dissolve in 20 mL water, add methylhydrazine (0.46 g, 10 mmol ), and stirred for 1 h. After the reaction, the water was drained to obtain a white mixture, which was extracted three times with 10 mL of acetonitrile, and the filtrates were combined and dried in vacuo to obtain a white solid, namely 5-(1 ' -methyl)hydrazinotetrazole. Yield: 58%.
[0030] The reaction formula is: BrCN+NaN 3 +CH 3 NHNH 2 à [CH 3 N(NH2 ) CN 4 H]
Embodiment 3
[0031] Embodiment 3,5-(1 ' -Methyl)hydrazino tetrazolium lithium salt
[0032] 2.28 g (20 mmol) 5-(1 ' -Methyl)hydrazinotetrazole was dissolved in 20 mL of water, 20 mL of an aqueous solution containing an equimolar amount (0.84 g, 20 mmol) of lithium hydroxide hydrate was added, and the reaction was fully stirred for 1 h. The solution was spin-dried in vacuo at 80 °C to obtain a white solid, namely 5-(1 ' - Methyl)hydrazinotetrazole lithium salt. Yield: 99%.
[0033] The reaction formula is: LiOH + [CH 3 N(NH 2 ) CN 4 H] à Li + [CH 3 N(NH 2 ) CN 4 ] - + H 2 o
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com