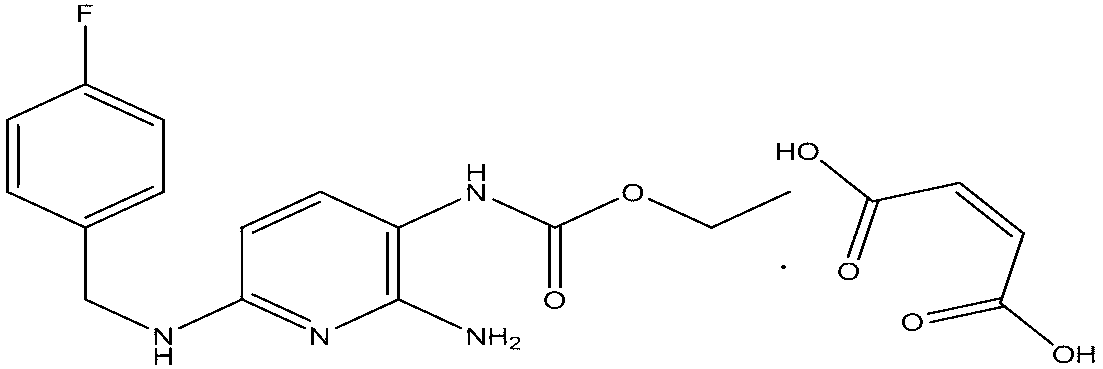
Capsule containing flupirtine maleate and preparation method thereof
A technology of flupirtine maleate and capsules, which is applied in the field of medicine, can solve the problems of difficult storage, poor stability, and low dissolution rate, and achieve the effect of convenient product storage and enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
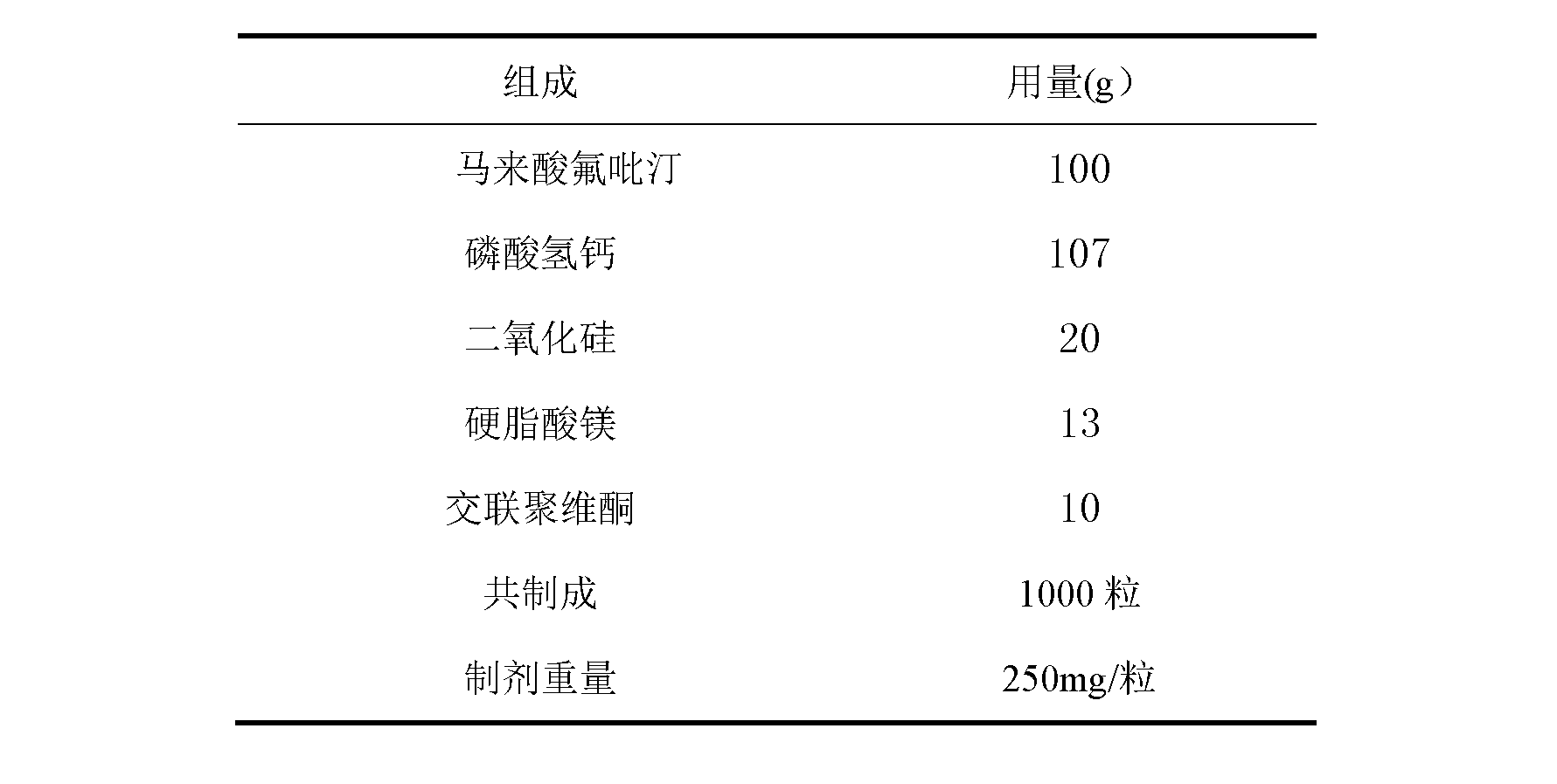
[0054] Embodiment 1, flupirtine maleate capsule prescription:
[0055]
[0056] Process:
[0057] 1) pass the calcium hydrogen phosphate through a 100-mesh sieve, and set aside;
[0058] 2) pulverize flupirtine maleate through a 600-mesh sieve, and set aside;
[0059] 3) mix flupirtine maleate with calcium hydrogen phosphate and 10g silicon dioxide, and 10g magnesium stearate;
[0060] 4) Put the above-mentioned obtained material in a dry granulator to make a dry material, and break it into dry granules through a swing granulator;
[0061] 5) passing the gained dry particles through 24 mesh and 80 mesh stainless steel sieves successively, collecting the part above the 80 mesh sieves as qualified particles;
[0062] 6) Add additional auxiliary materials crospovidone, 10g silicon dioxide, and 3g magnesium stearate to the above materials, and mix well;
[0063] 7) Capsule.
Embodiment 2
[0064] Embodiment 2, flupirtine maleate capsule prescription:
[0065]
[0066] Process:
[0067] 1) Pass calcium hydrogen phosphate through a 100-mesh sieve and set aside;
[0068] 2) Pulverize the flupirtine maleate through a 600-mesh sieve and set aside;
[0069] 3) Mix flupirtine maleate with calcium hydrogen phosphate, 10g silicon dioxide, and 10g magnesium stearate;
[0070] 4) Put the above-mentioned obtained material in a dry granulator to make a dry material, and crush it into dry granules through a swing granulator;
[0071] 5) Pass the obtained dry particles through 24-mesh and 80-mesh stainless steel screens in turn, and collect the part above the 80-mesh screen as qualified particles;
[0072] 6) Add external auxiliary materials crospovidone, 10g silicon dioxide, 3g magnesium stearate to the above materials, and mix well;
[0073] 7) Capsules.
Embodiment 3
[0074] Embodiment 3, flupirtine maleate capsule prescription:
[0075]
[0076] 1) Pass calcium hydrogen phosphate through a 100-mesh sieve and set aside;
[0077] 2) Pulverize the flupirtine maleate through a 600-mesh sieve and set aside;
[0078] 3) Mix flupirtine maleate with calcium hydrogen phosphate, 20g silicon dioxide, and 20g magnesium stearate;
[0079] 4) Put the above-mentioned obtained materials in a dry granulator to make dry materials, and crush them into dry granules through a swing granulator;
[0080] 5) Pass the obtained dry particles through 24-mesh and 80-mesh stainless steel screens in turn, and collect the part above the 80-mesh screen as qualified particles;
[0081] 6) Add external auxiliary materials crospovidone, 20g silicon dioxide, 6g magnesium stearate to the above materials, and mix well;
[0082] 7) Capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com