Method for synthesizing MIL-101 chrome metal organic framework by nitrogen heterocyclic
A nitrogen heterocyclic compound, organic framework technology, applied in chemical instruments and methods, compounds containing periodic table Group 6/16 elements, organic chemistry, etc. High, high-purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
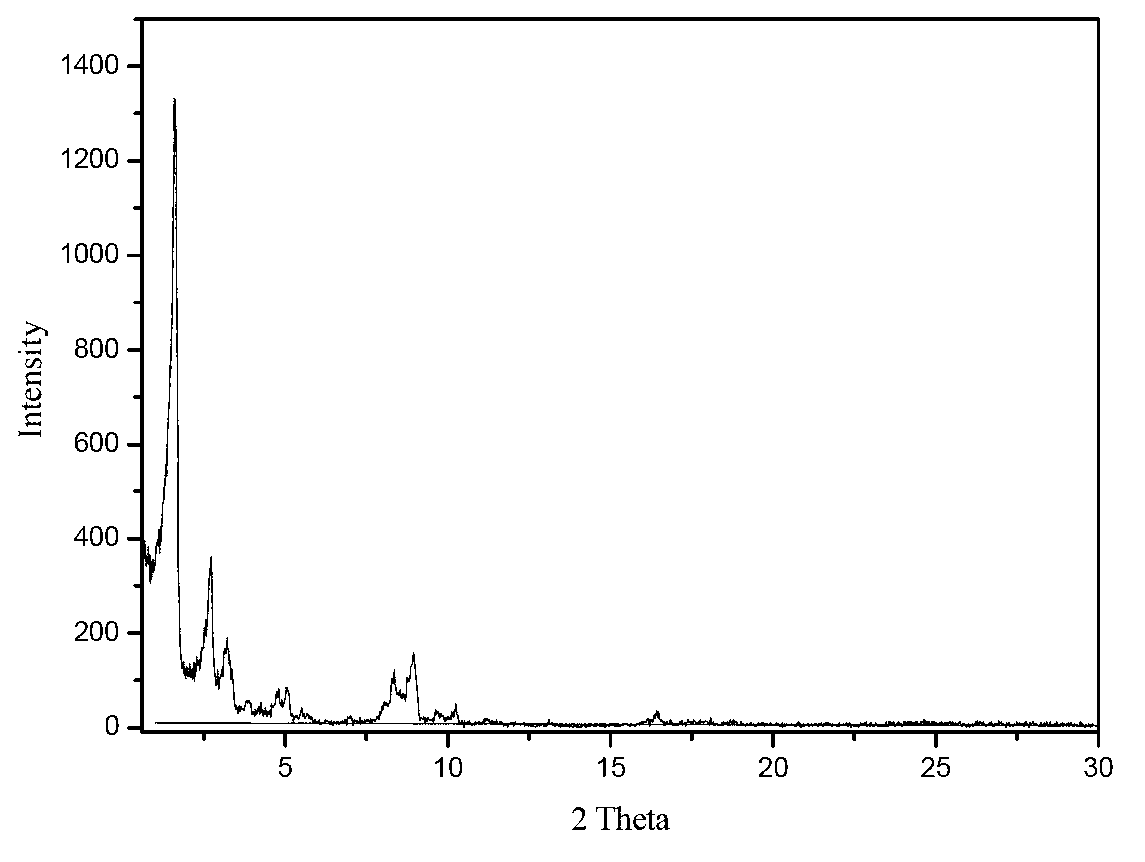
[0052] Add 0.1809g of 4-nitroimidazole into 80ml of deionized water, place it on a magnetic stirrer and stir for 15min, then add 2.6581g of terephthalic acid, stir for 15min, finally add 6.4024gCr(NO3)3·9H2O, and continue stirring until the nitric acid Chromium dissolves. The mixed solution was transferred into a 110ml stainless steel autoclave lined with polytetrafluoroethylene. The reaction kettle was placed in a blast drying oven, the temperature was raised to 150°C and kept for 24 hours, and after cooling down to room temperature, the product was taken out from the kettle, washed with deionized water, filtered with suction, and dried at 100°C to obtain 0.8 g of the product with a yield of 15%.
Embodiment 2
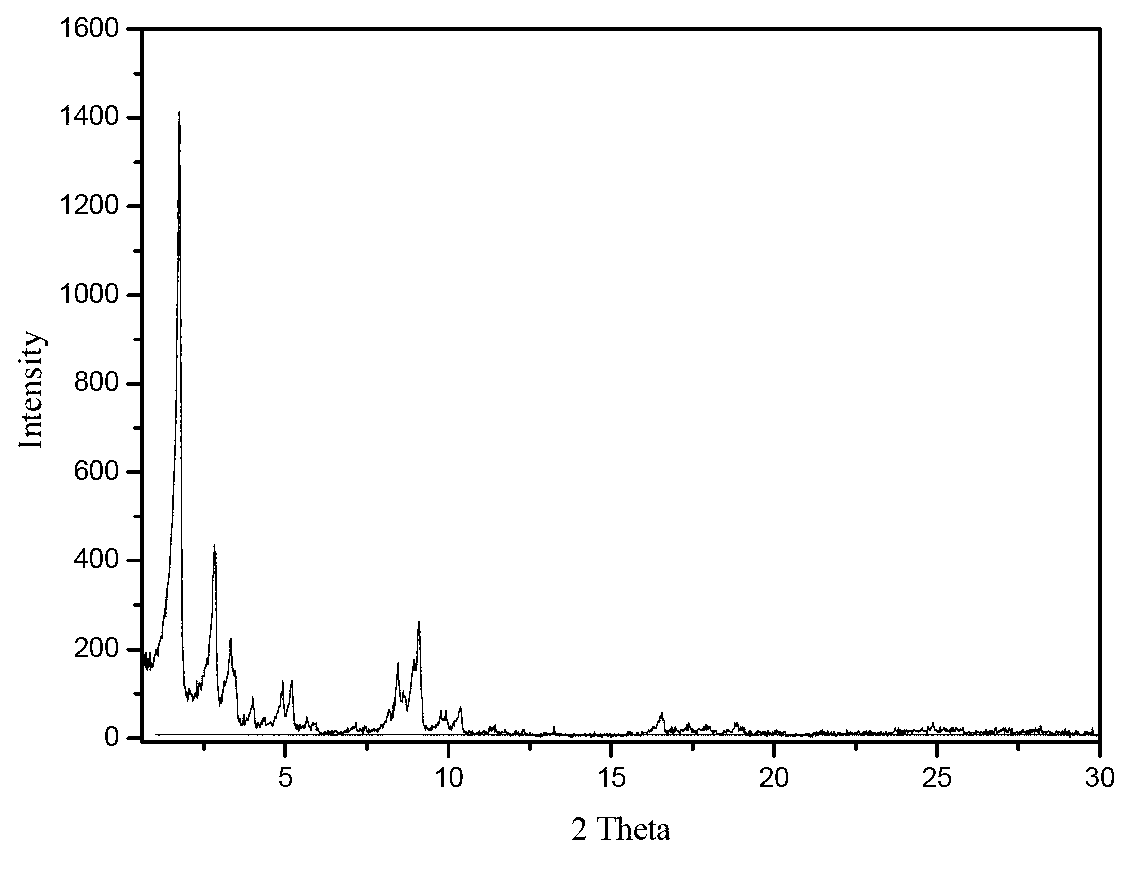
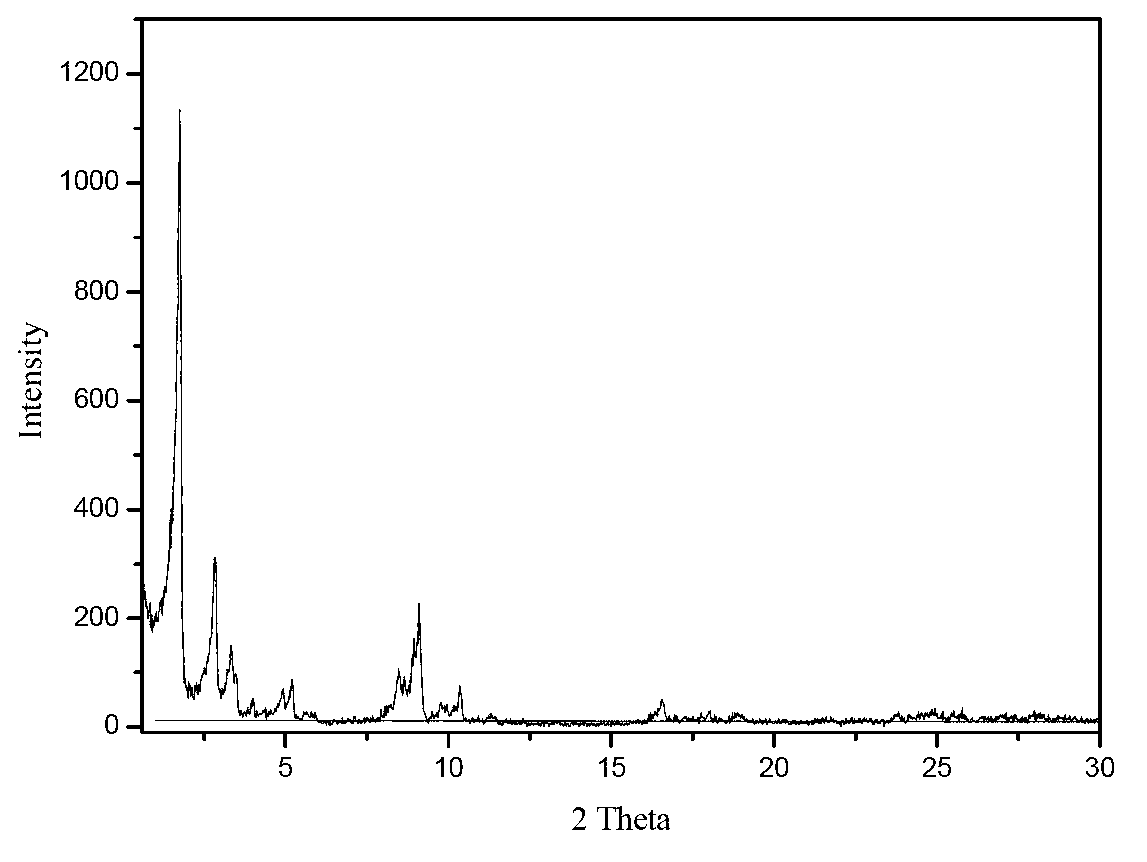
[0054] Add 0.9046g of 4-nitroimidazole into 80ml of deionized water, place it on a magnetic stirrer and stir for 15min, then add 2.6581g of terephthalic acid, stir for 15min, finally add 6.4024gCr(NO3)3·9H2O, and continue stirring until the nitric acid Chromium dissolves. The mixed solution was transferred into a 110ml stainless steel autoclave lined with polytetrafluoroethylene. The reaction kettle was placed in a blast drying oven, heated to 150°C and kept for 24 hours. After cooling down to room temperature, the product was taken out from the kettle, washed with deionized water, filtered with suction, and dried at 100°C to obtain 2.9 g of the product. The yield was 59%. There are no characteristic peaks of terephthalic acid crystals in the XRD spectrum.
Embodiment 3
[0056] Add 1.2665g of 4-nitroimidazole to 80ml of deionized water, place it on a magnetic stirrer and stir for 15min, then add 2.6581g of terephthalic acid, stir for 15min, finally add 6.4024g of Cr(NO3)3 9H2O, and continue stirring until the nitric acid Chromium dissolves. The mixed solution was transferred into a 110ml stainless steel autoclave lined with polytetrafluoroethylene. The reaction kettle was placed in a blast drying oven, the temperature was raised to 150°C and kept for 24 hours, and after cooling down to room temperature, the product was taken out from the kettle, washed with deionized water, filtered with suction, and dried at 100°C to obtain 3.8 g of the product with a yield of 74%. There are no characteristic peaks of terephthalic acid crystals in the XRD spectrum
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com