Zinc oxide/acid containing compositions and methods for treating and/or preventing enzymatic irritation
A composition, zinc oxide technology, applied in the direction of medical preparations containing active ingredients, anhydride/acid/halide active ingredients, drug combinations, etc., can solve problems such as inflammation and antimicrobial barrier defects, permeability barrier dysfunction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
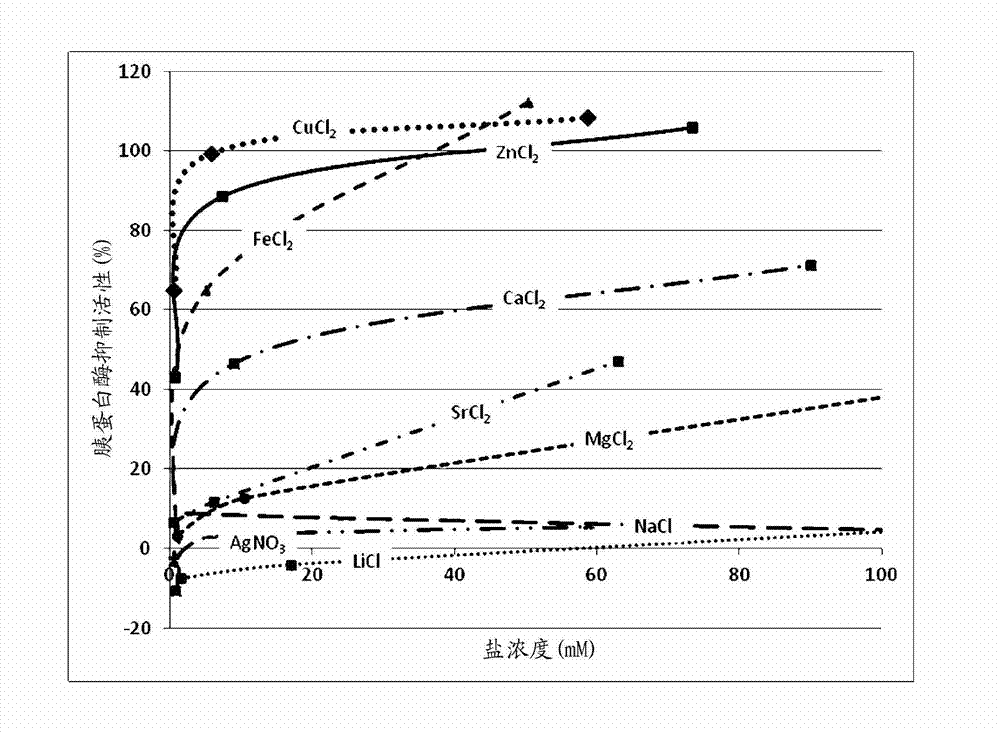
[0080] Effect of Cations on Trypsin Inhibition .
[0081] The purpose of the following experiments was to determine whether and to what extent the following cations inhibit trypsin activity in an in vitro assay.
[0082] Inhibition of Trypsin-Induced Cleavage of Fluorescent Casein Peptides Using EnzChek TM Protease assay kit, according to the manufacturer's instructions (revised EnzChek TM Protease Assay Kit Product Information; Molecular Probes, Eugene OR) Measurements were performed Briefly, different concentrations of test material were prepared in 1x phosphate buffered saline (PBS, pH 7.4). Prepare a trypsin working solution (Sigma, St. Louis, MO, units / mL) in the digestion buffer provided in the assay kit. Prepare a stock solution of BODIPY FL casein (trypsin substrate, mg / mL) by adding 0.2 mL to the substrate vial (supplied in the kit) and prepare the final substrate by diluting in digestion buffer (pH 7.8) Working solution (10 µg / mL). After incubating trypsin (wit...
example 2
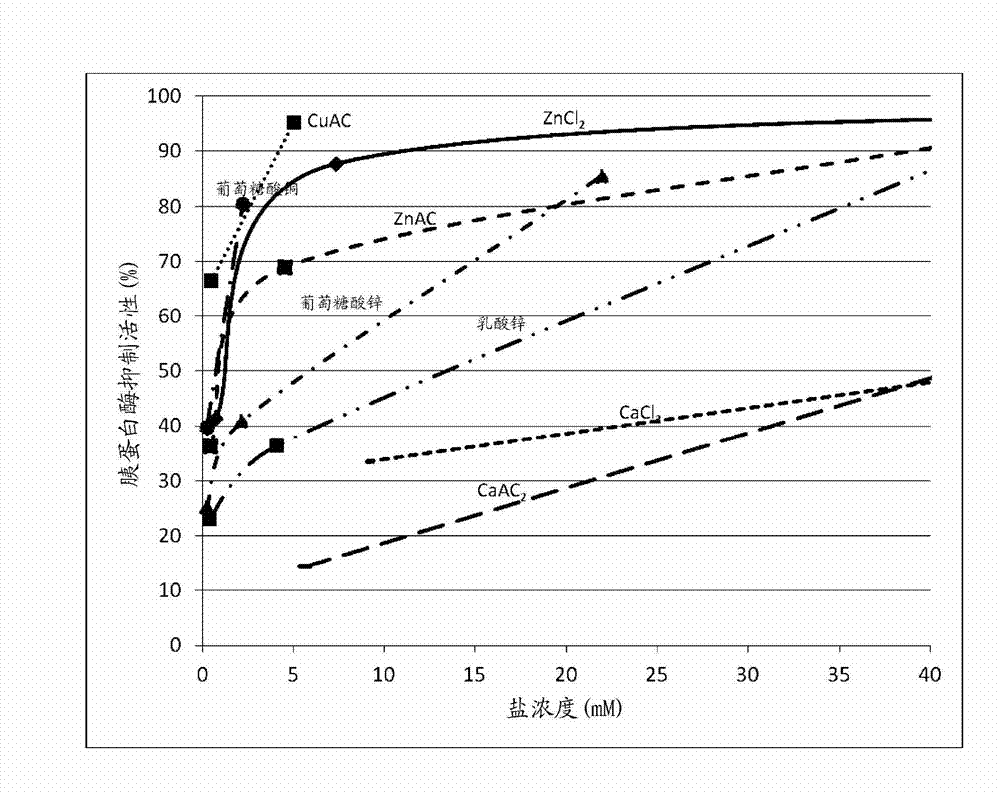
[0087] Example 2: Effect of Salt Forms of the Same Divalent Cation on Trypsin Inhibition .
[0088] The goal of the following experiments was to determine whether and to what extent different salt forms of the same divalent cation had an effect on the inhibition of trypsin activity in an in vitro assay.
[0089] Trypsin inhibitory activity was assayed as described in Example 1 and the effect of different salt forms of the same cation on trypsin inhibitory activity was assessed. Table 2 shows the molecular weight, concentration and trypsin inhibitory activity of the test compounds.
[0090] Table 2
[0091]
[0092]
[0093] The results obtained indicate that different salt forms of similar cations have different potencies in trypsin inhibitory activity.
[0094] As shown in Table 2, Zn in the chloride salt form when calibrated with molar concentration 2+ Has the highest trypsin inhibitory activity, followed by acetate, gluconate and lactate forms. Table 2 also sh...
example 3
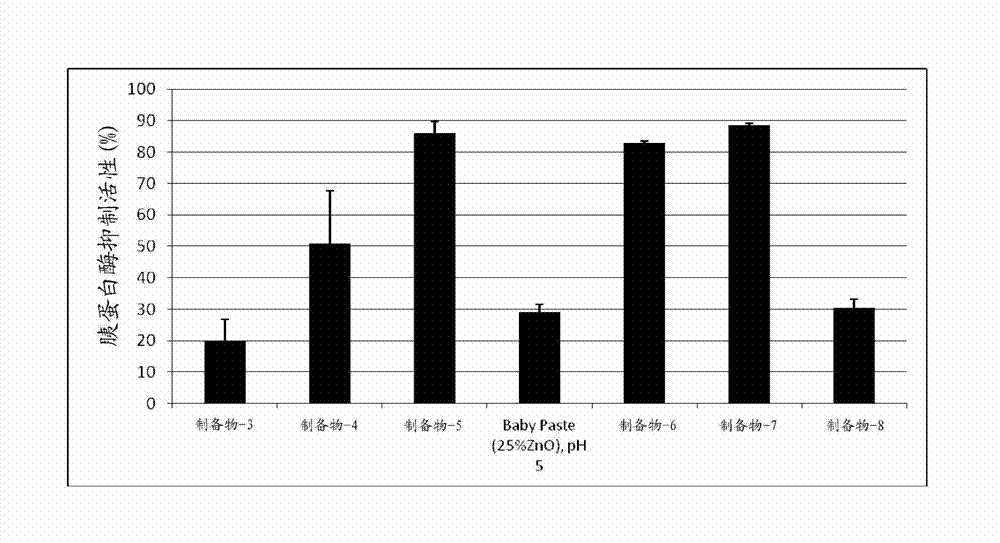
[0096] The purpose of the following experiments was to determine whether and to what extent the addition of divalent cations to talc inhibited trypsin activity in an in vitro assay. Inhibition of Trypsin-Induced Cleavage of Fluorescent Casein Peptides Using EnzChek TM Protease assay kit, according to the manufacturer's instructions (revised EnzChek TM Protease Assay Kit Product Information; Molecular Probes, Eugene OR) for measurement. Briefly, talc stock solutions were prepared in 1x phosphate buffered saline (PBS, pH 7.4) with (1) different concentrations of divalent cation salts (as indicated in Table 3) and with (2) different concentrations of magnesium chloride (as indicated in Table 4). Prepare a trypsin working solution (Sigma, St. Louis, MO, units / mL) in the digestion buffer provided in the assay kit. Prepare a stock solution of BODIPY FL casein (trypsin substrate, mg / mL) by adding 0.2 mL to the substrate vial (supplied in the kit) and prepare the final substrate by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com