Ambroxol hydrochloride composition and preparation thereof
A technology of ambroxol hydrochloride and its composition, which is applied in the field of medicine, can solve problems such as complex formulas and increased hidden dangers of drug safety, and achieve the effect of simple operation and enhanced safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
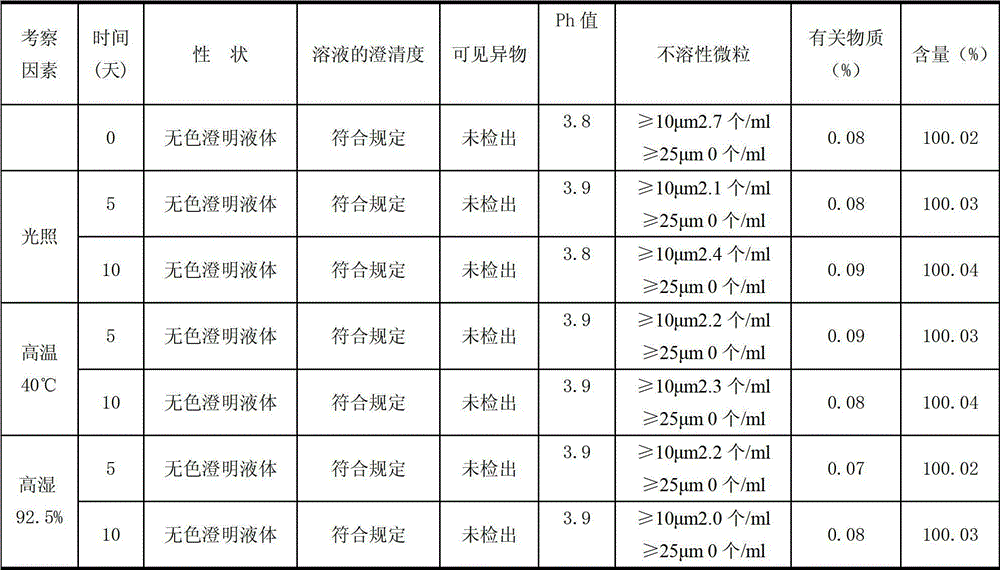
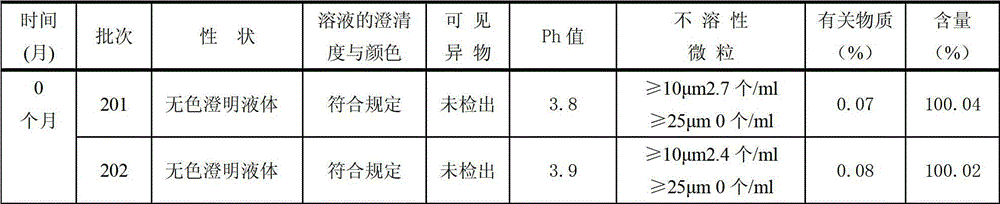
[0028] An ambroxol hydrochloride composition comprises: 15 parts by weight of ambroxol hydrochloride, 50 parts by weight of mannitol, 1 part by weight of meglumine, and 0.05 parts by weight of disodium edetate.
[0029] The dosage form of the composition is injection, and each injection contains: 15 mg of ambroxol hydrochloride, 50 mg of mannitol, 1-mg of meglumine, 0.05 mg of disodium edetate, and 2 ml of water for injection.
[0030] The preparation method of the preparation is as follows: dissolve the prescribed amount of meglumine and mannitol in water for injection with a volume ratio of 80%, and heat to 35° C., then add the prescribed amount of ambroxol hydrochloride, stir evenly, and then Add the prescribed amount of disodium edetate; add medical activated carbon at 30°C, filter, then cool to room temperature; add water for injection to the full amount, filter with a 0.22 μm pore size filter membrane, cool the filtrate to 3°C and use The 0.22 μm pore size filter membran...
Embodiment 2
[0032] An ambroxol hydrochloride composition comprises: 15 parts by weight of ambroxol hydrochloride, 150 parts by weight of mannitol, 1 part by weight of meglumine, and 0.5 parts by weight of disodium edetate.
[0033] The dosage form of the composition is injection, and each injection contains: 15 mg of ambroxol hydrochloride, 150 mg of mannitol, 10 mg of meglumine, 0.5 mg of disodium edetate, and 2 ml of water for injection.
[0034] The preparation method of the preparation is as follows: dissolve the prescribed amount of meglumine and mannitol in water for injection with a volume ratio of 70%, and heat to 35°C, then add the prescribed amount of ambroxol hydrochloride, stir well, and then Add the prescribed amount of disodium edetate; add medical activated carbon at 30°C, filter, then cool to room temperature; add water for injection to the full amount, filter with a 0.22 μm pore size filter membrane, cool the filtrate to 5°C and use The 0.22 μm pore size filter membrane i...
Embodiment 3
[0036] An ambroxol hydrochloride composition comprises: 15 parts by weight of ambroxol hydrochloride, 80 parts by weight of mannitol, 2 parts by weight of meglumine, and 0.08 parts by weight of disodium edetate.
[0037] The dosage form of the composition is injection, and each injection contains: 15 mg of ambroxol hydrochloride, 80 mg of mannitol, 2 mg of meglumine, 0.08 mg of disodium edetate, and 2 ml of water for injection.
[0038] The preparation method of the preparation is as follows: dissolve the prescribed amount of meglumine and mannitol in water for injection with a volume ratio of 70%, and heat to 30°C, then add the prescribed amount of ambroxol hydrochloride, stir evenly, and then Add the prescribed amount of disodium edetate; add medical activated carbon at 35°C, filter, then cool to room temperature; add water for injection to the full amount, filter with a 0.22 μm pore size filter membrane, cool the filtrate to 0°C and use The 0.22 μm pore size filter membrane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com