Organic thiosulfuric acid derivative preparation method
A technology of organic thiosulfuric acid and sodium thiosulfate is applied in the field of preparation of organic thiosulfuric acid derivatives to achieve the effects of avoiding post-treatment, developing prospects and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
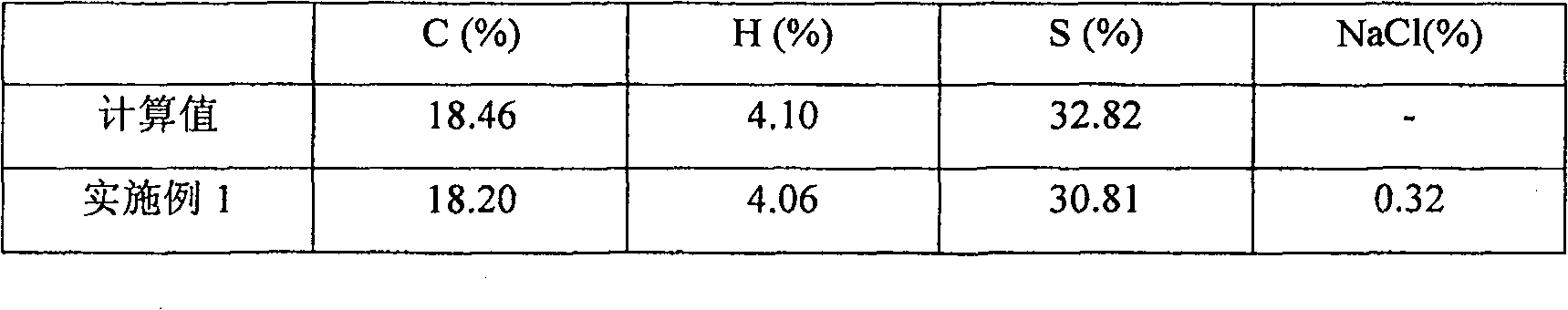
[0024] A 200mL four-necked flask equipped with a mechanical stirring device, a thermometer and a reflux condenser was used as a reaction vessel, and 12.4g of sodium thiosulfate pentahydrate was weighed in the four-necked flask, and 100mL of water was added into the four-necked flask with mechanical stirring to dissolve, and 3.9 g1,6-dichlorohexane, heat up the above solution, and when the temperature of the reaction solution rises to 100°C, slowly add 1% sodium hydroxide solution through a peristaltic pump to adjust the pH value of the reaction solution to 7.5. After stirring and reacting for 6 hours, stop adding the sodium hydroxide solution, add dropwise a 0.05% hydrochloric acid solution to adjust the pH value of the solution to 7, pour out the solution, filter and leave it at 4°C for 3 hours, and a large amount of solids will precipitate out. The solid product was dried at 45°C to obtain the disodium salt of hexamethylene 1,6-dithiosulfate dihydrate.
Embodiment 2
[0026] A 200mL four-necked flask equipped with a mechanical stirring device, a thermometer and a reflux condenser was used as a reaction vessel, and 24.8g of sodium thiosulfate pentahydrate was weighed in the four-necked flask, and 100mL of water was added into the four-necked flask with mechanical stirring to dissolve, and 7.4 g1,6-dichlorohexane, heat up the above solution, and when the temperature of the reaction solution rises to 90°C, slowly add 1.2% sodium hydroxide solution through a peristaltic pump to adjust the pH value of the reaction solution to 7. After stirring and reacting for 9 hours, stop adding the sodium hydroxide solution, add dropwise a 0.5% hydrochloric acid solution to adjust the pH value of the solution to 6.5, pour the solution out, filter and stand at 0°C for 1 hour, a large amount of solids precipitate out, and filter out The solid product was dried at 55°C to obtain the disodium salt of hexamethylene 1,6-dithiosulfate dihydrate.
Embodiment 3
[0028] A 200mL four-necked flask equipped with a mechanical stirring device, a thermometer and a reflux condenser was used as a reaction vessel, and 52.1g of sodium thiosulfate pentahydrate was weighed in the four-necked flask, and 100mL of water was added to the four-necked flask with mechanical stirring to dissolve, while stirring, 14.8 g1,6-dichlorohexane, heat up the above solution, and when the temperature of the reaction solution rises to 115°C, slowly add 2% sodium hydroxide solution through a peristaltic pump to adjust the pH value of the reaction solution to 9. After stirring and reacting for 6 hours, stop adding the sodium hydroxide solution, add dropwise a 0.1% hydrochloric acid solution to adjust the pH value of the solution to 7, pour the solution out, filter and leave it standing at 5°C for 1 hour, and a large amount of solids precipitate out. The solid product was dried at 45°C to obtain the disodium salt of hexamethylene 1,6-dithiosulfate dihydrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com