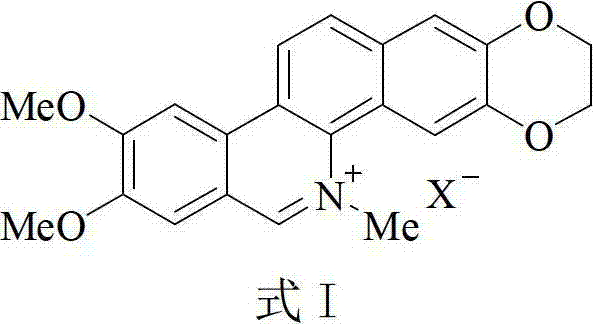
2,3-dioxyethyl-5-methyl-8,9-dimethoxy benzophenanthridine derivative, and preparation method and application thereof
A technology of dimethoxytriphenanthridine and dioxyethyl is applied in the field of preparing anti-tumor pharmaceutical compositions, which can solve the problem of low tumor cell selectivity and achieve good anti-tumor activity and significant cytotoxic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
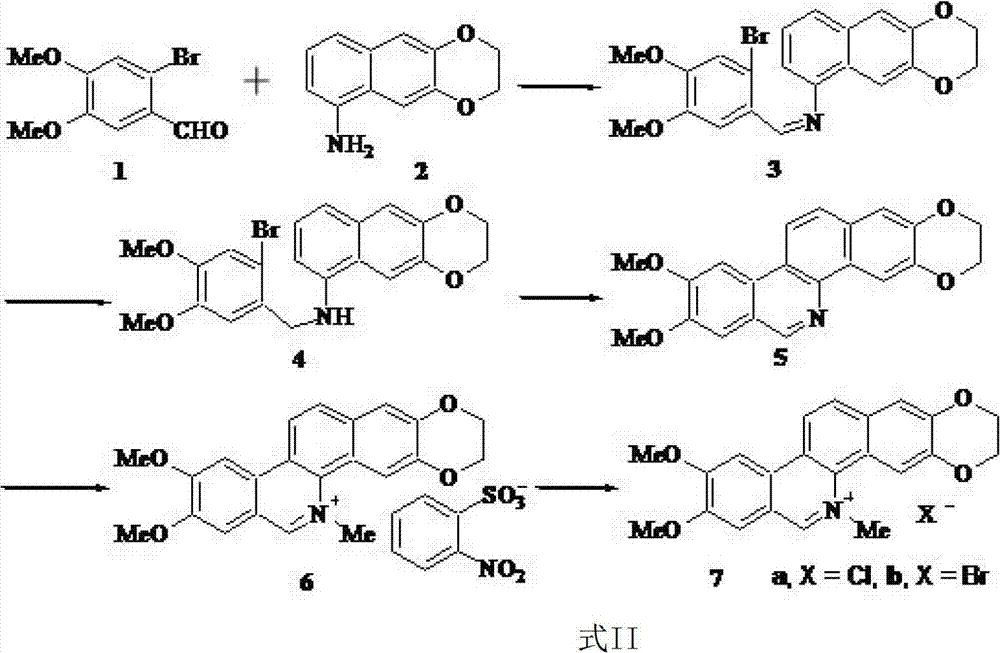
Examples
Embodiment 1
[0016] Example 1: Synthesis of 2-bromo-4,5-dimethoxybenzaldehyde (compound 1)
[0017] Add methanol (20mL) and 3,4-dimethoxybenzaldehyde (1.42g, 8.55mmol) into a round bottom flask, slowly add liquid bromine (0.5mL, 9.76mmol), react for 1 hour, and remove by rotary evaporation solvent. Water (50 mL) was added under constant stirring, and the resulting paste was suction-filtered with a Buchner funnel, washed with water and methanol, and dried to obtain 1.76 g of a colorless to slightly yellow product, with a yield of 84%. M.p.143~145℃, 1 H NMR (500MHz, CDCl 3 ) δ: 10.21 (s, 1H, CHO), 7.44 (s, 1H, ArH), 7.08 (s, 1H, ArH), 3.97 (s, 3H, OCH 3 ), 3.94 (s, 3H, OCH 3 ). The structural formula of compound 1 is as follows:
[0018]
Embodiment 2
[0019] Example 2: Synthesis of 2,3-dioxoethyl-5-aminonaphthalene (compound 2)
[0020] Add absolute ethanol (170mL), 2,3-dioxoethyl-5-nitronaphthalene (1.56g, 7.18mmol) and 0.15g of 10% Pd / C into a round-bottomed flask, and pass through hydrogen to react 8 hours, filtered to remove ethanol, and the resulting residue was purified by silica gel column chromatography to obtain 1.11 g of lavender solid, with a yield of 82%. M.p.169~173℃, 1 H NMR (500MHz, CDCl 3 ) δ: 7.28 (s, 1H, ArH), 7.26 (s, 1H, ArH), 7.18 (m, 2H, ArH), 6.65 (m, 1H, ArH), 4.36 (s, 4H, OCH 2 CH 2 O), 3.97 (s, 2H, NH 2 ), 13 C NMR (CDCl 3 , 125MHz) δ: 143.92, 143.28, 140.94, 130.62, 124.78, 119.90, 117.80, 113.25, 108.20, 106.54 (10C, 2Ar), 64.54, 64.52 (2C, OCH 2 CH 2 O); IR (KBr) ν: 3838 (N-H), 3735 (N-H), 3368, 1636 (Ar-H), 1517 (Ar-H), 1475 (Ar-H), 1290 (C-O), 1252 (C-O ), 1067 (C-O), 904, 864cm -1 ;APCIMS m / z:201.83[M+H] + . The structural formula of compound 2 is as follows:
[0021]
Embodiment 3
[0022] Example 3: Synthesis of N-(2-bromo-4,5-dimethoxybenzylidene)-6,7-(dioxyethyl)-1-naphthylamine (Compound 3)
[0023] Add absolute ethanol (150mL), compound 1 (2.19g, 8.97mol) and compound 2 (1.68g, 8.97mol) into a round bottom flask, heat for 10 hours, filter after cooling, wash with ethanol, and dry 3.05 g of a khaki solid were obtained, yield 82%. M.p.218~221℃, 1 H NMR (500MHz, CDCl 3 ) δ: 8.81 (s, 1H, CHN), 7.94 (s, 1H, ArH), 7.76 (s, 1H, ArH), 7.57 (d, J=8.2Hz, 1H, ArH), 7.33 (m, 2H, ArH), 7.09 (s, 1H, ArH), 6.95 (d, J=7.2Hz, 1H, ArH), 4.37 (brs, 4H, OCH 2 CH 2 O), 4.03 (s, 3H, OCH 3 ), 3.97 (s, 3H, OCH 3 ); 13 C NMR (CDCl 3 , 125MHz) δ: 158.40 (1C, CHN), 152.33, 148.87, 148.01, 144.38, 143.74, 130.29, 127.42, 125.08, 124.53, 118.03, 115.51, 115.22, 112.49, 111.43, 110.439 (1, Ar, 110.43) (2C, OCH 2 CH 2 O), 56.32, 56.23 (2C, 2OCH 3 ); IR (KBr) ν: 1592 (Ar-H), 1564 (Ar-H), 1505 (Ar-H), 1465 (Ar-H), 1288 (C-O), 1248 (C-O), 1214 (C-O) , 900, 862cm -1 ;APC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com