Method for preparing 1, 2-cyclohexane dioctyl phthalate diisooctyl by hydrogenation
A technology of diisooctyl cyclohexanedicarboxylate and diisooctyl phthalate is applied in the field of hydrogenation to prepare diisooctyl cyclohexanedicarboxylate, and can solve the problem of high reaction temperature and conversion problems such as low rate or selectivity, to achieve the effect of reducing reaction temperature and energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
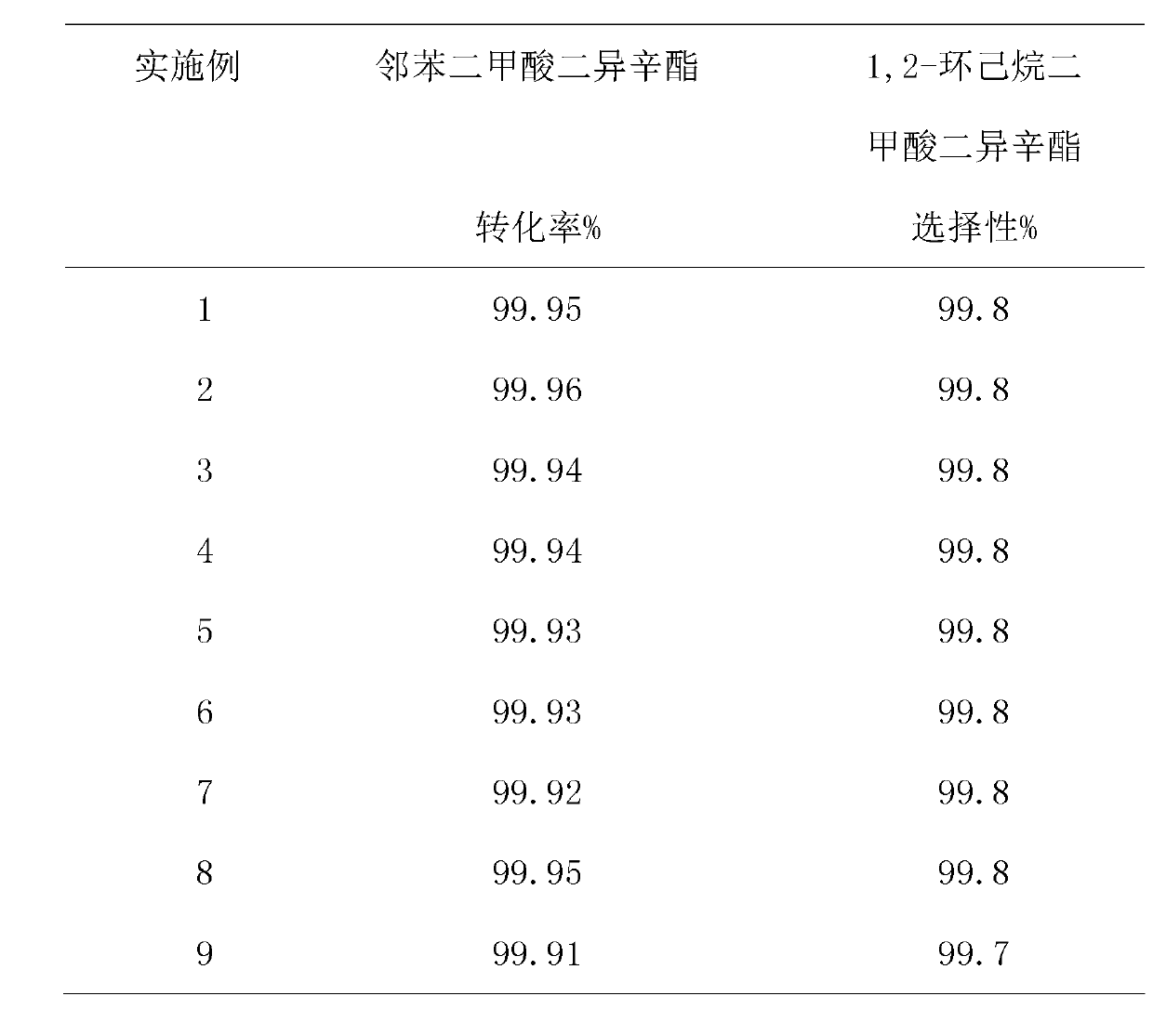
Examples
Embodiment 1
[0021] The catalyst used is Pd-Ru / Al 2 o 3 . The weight composition of the catalyst is: Pd=0.5%, Ru=1%, and the rest is carrier Al 2 o 3 . The catalyst active components Pd and Ru were supported on the carrier Al by conventional impregnation method. 2 o 3 superior. 10 grams of catalyst is activated by hydrogen before the reaction, the activation conditions are: GHSV=1000~2000h -1 , Atmospheric pressure ~1.0MPa, 250~450℃, reduction time 5~12 hours. A trickle bed reactor is used. The reaction temperature is 175°C, the hydrogen pressure is 15MPa, the H2 / ester molar ratio is 600, and the volumetric space velocity of diisooctyl phthalate is 0.4h -1 ,, Reaction time 24h, sampling analysis. The analysis of products and raw materials adopts gas chromatography, and the specific conditions are: nitrogen is the carrier gas, the flow rate is 1.5mL / min., and the injection volume is 4 microliters; FID hydrogen flame ion detector, HP-5 column, column temperature 260°C.
Embodiment 2
[0023] The volume space velocity of diisooctyl phthalate is 0.3h -1 , other conditions are identical with embodiment 1.
Embodiment 3
[0025] The volume space velocity of diisooctyl phthalate is 0.5h -1 , other conditions are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com