Novel polymorphs of dasatinib, and preparation method thereof
A polymorph, dasatinib technology, applied in the field of new polymorph and its preparation, can solve the problem of poor solubility, difficult to effectively improve product purity, affecting the stability and bioavailability of raw materials and preparations And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
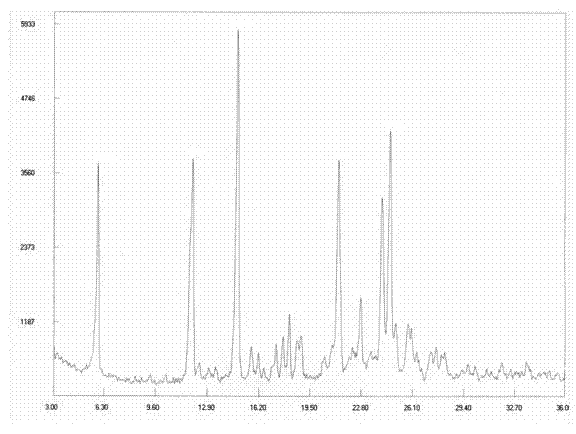
[0163] Example 1: Preparation of Dasatinib Polymorph I
[0164] Take 10g of Dasatinib crude product and place it in a reaction flask, add a mixed solvent of 60ml DMF and 120ml isopropanol, heat up to 70-80°C under stirring to dissolve, slowly cool to 50-60°C and stir for 1 hour, Crystallize at 30°C for 1 hour, and finally lower the temperature to 5-10°C to fully separate out the solid and grow the crystal for 2 hours, filter with suction, wash the filter cake with isopropanol and drain it. The solid was dried at 50°C under reduced pressure (-0.09 MPa) to obtain 8.1 g of white solid, yield 81%. The purity of related substances is 99.95%.
Embodiment 2
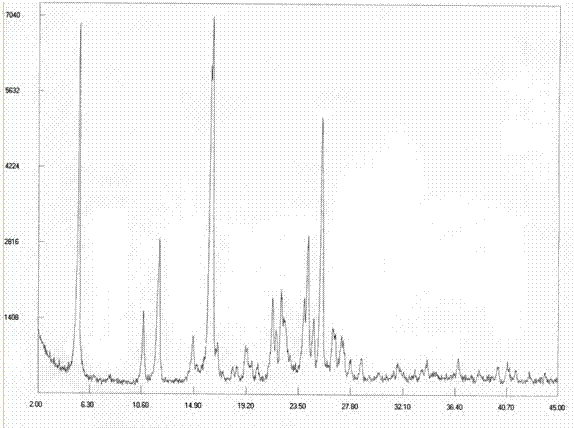
[0165] Example 2: Preparation of Dasatinib Polymorph I
[0166] Take 10g of Dasatinib crude product and place it in a reaction flask, add a mixed solvent of 50ml DMSO and 150ml isopropanol, heat up to 70-80°C under stirring to dissolve, slowly cool to 50-60°C and stir for 1 hour, then in 20-60°C Crystallize at 30°C for 1 hour, and finally lower the temperature to 5-10°C to fully separate out the solid and grow the crystal for 3 hours, filter with suction, wash the filter cake with isopropanol and drain it. The solid was dried at 50°C under reduced pressure (-0.09MPa) to obtain 8.3g of white solid, yield 83%. The purity of the related substance is 99.94%.
Embodiment 3
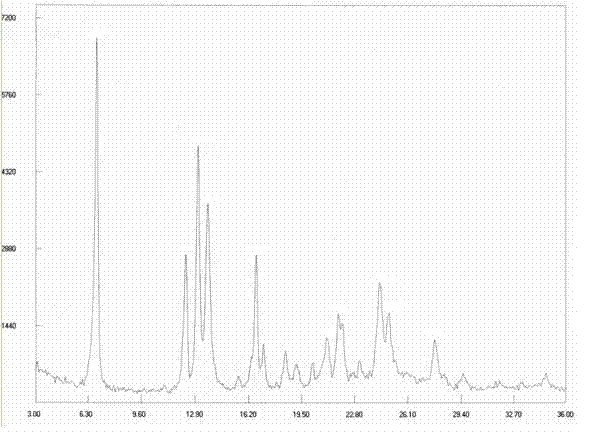
[0167] Example 3: Preparation of Dasatinib Polymorph I
[0168] Take 1g of Dasatinib crude product and place it in a reaction bottle, add a mixed solvent of 5ml DMF and 5ml isopropanol, heat up to 70-80°C under stirring to dissolve, slowly cool to 50-60°C and stir for 1 hour, then in 20-60°C Crystallize at 30°C for 1 hour, and finally lower the temperature to 5-10°C to fully separate out the solid and grow the crystal for 2 hours, filter with suction, wash the filter cake with isopropanol and drain it. The solid was dried at 50°C under reduced pressure (-0.09MPa) to obtain 0.84g of a white solid, with a yield of 84%. The purity of the related substance is 99.93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com