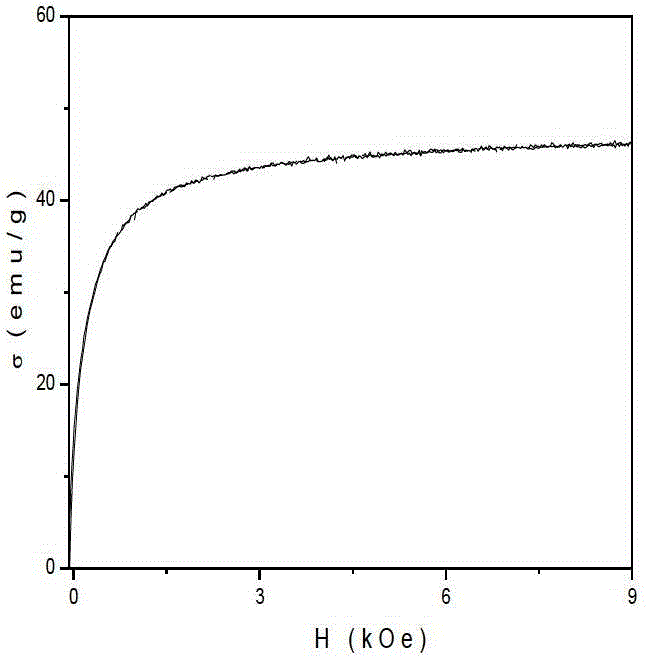
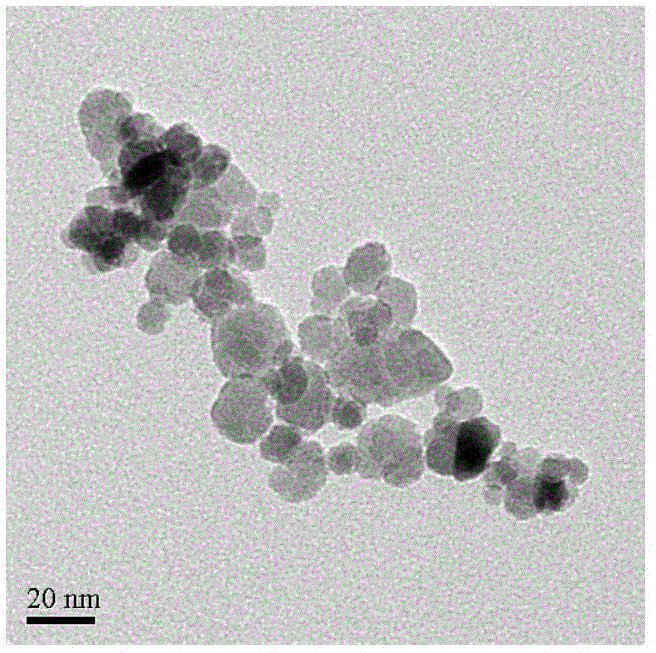
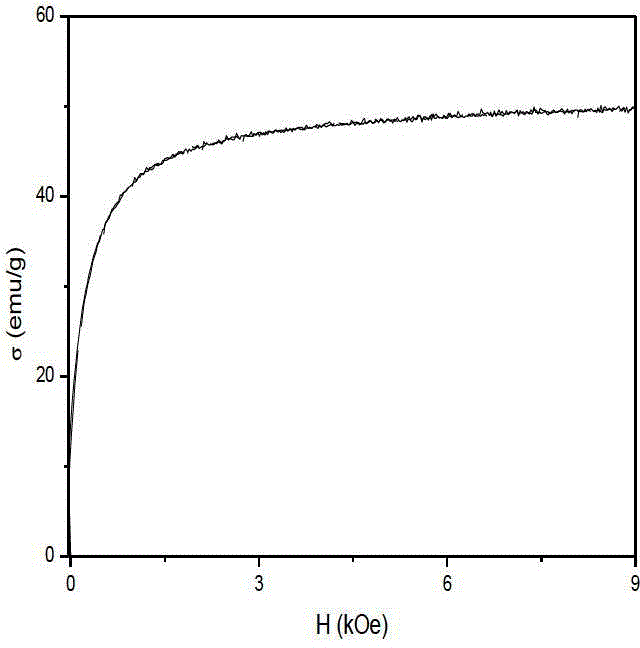
Preparation method of gamma-Fe2O3/ZnFe2O4 magnetic composite nano particle
A magnetic composite nano, -fe2o3 technology, applied in the field of nanomaterials, to achieve the effect of low raw material cost, small particle size, and easy preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1. Preparation of hydroxide precursor:
[0054] 1) Preparation of FeCl 3 Aqueous solution (40ml, 1mol / L) is solution a; prepare Mg(NO 3 ) 2 Aqueous solution (10ml, 2mol / L), add HCl (4.2mL, 12mol / L) to obtain solution b; prepare NaOH aqueous solution (500mL, 0.7mol / L) as solution c.
[0055] 2) Mix the solution a with the solution b to prepare FeCl 3 / Mg(NO 3 ) 2 Mixed solution, i.e. solution d;
[0056] 3) Quickly pour the solution d into the solution c under stirring to prepare the reaction solution e;
[0057] 4) Heat the reaction liquid e to boiling on the electric stove, keep boiling for 5 minutes, and then stop heating;
[0058] 5) The reaction liquid e was removed from the electric furnace, and naturally cooled to room temperature. After about two hours, the reddish-brown precipitated hydroxide precursor was completely precipitated.
[0059] 2. Preparation of Magnetic Composite Nanoparticles
[0060] 1) Preparation of FeCl 2 Aqueous solution (400mL, 0.25...
Embodiment 2
[0070] 1. Preparation of hydroxide precursor:
[0071] 1) Preparation of FeCl 3 Aqueous solution (40ml, 1mol / L) is solution a; prepare Mg(NO 3 ) 2 Aqueous solution (10ml, 2mol / L), add HCl (4.2mL, 12mol / L) to obtain solution b; prepare NaOH aqueous solution (500mL, 0.7mol / L) as solution c;
[0072] 2) Mix the solution a with the solution b to prepare FeCl 3 / Mg(NO 3 ) 2 Mixed solution, i.e. solution d;
[0073] 3) Quickly pour the solution d into the solution c under stirring to prepare the reaction solution e;
[0074] 4) Heat the reaction liquid e to boiling on the electric stove, keep boiling for 5 minutes, and then stop heating;
[0075] 5) The reaction liquid e was removed from the electric furnace, and naturally cooled to room temperature. After about two hours, the reddish-brown precipitated hydroxide precursor was completely precipitated.
[0076] 2. Preparation of Magnetic Composite Nanoparticles
[0077] 1) Preparation of FeCl 2 Aqueous solution (400mL, 0.25...
Embodiment 3
[0087] 1. Preparation of hydroxide precursor:
[0088] 1) Preparation of FeCl 3 Aqueous solution (40ml, 1mol / L) is solution a; prepare Mg(NO 3 ) 2 Aqueous solution (10ml, 2mol / L), add HCl (4.2mL, 12mol / L) to obtain solution b; prepare NaOH aqueous solution (500mL, 0.7mol / L) as solution c;
[0089] 2) Mix the solution a with the solution b to prepare FeCl 3 / Mg(NO 3 ) 2 Mixed solution, i.e. solution d;
[0090] 3) quickly pour the solution d into the solution c under stirring to prepare the reaction solution e;
[0091] 4) Heating the reaction solution e to boiling on an electric furnace, keeping boiling for 5 minutes, and then stopping heating;
[0092] 5) The reaction liquid e was removed from the electric furnace, and naturally cooled to room temperature. After about two hours, the reddish-brown precipitated hydroxide precursor was completely precipitated.
[0093] 2. Preparation of Magnetic Composite Nanoparticles
[0094] 1) Preparation of FeCl 2 Aqueous solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com