Method for preparing isononyl olefine aldehyde
A technology of alkenal and isononyl, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of using sodium hydroxide or potassium hydroxide aqueous solution, and achieve simple equipment and high production efficiency The effect of low price and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
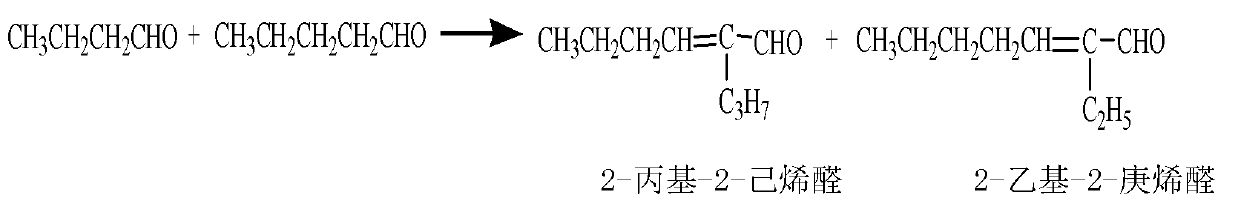
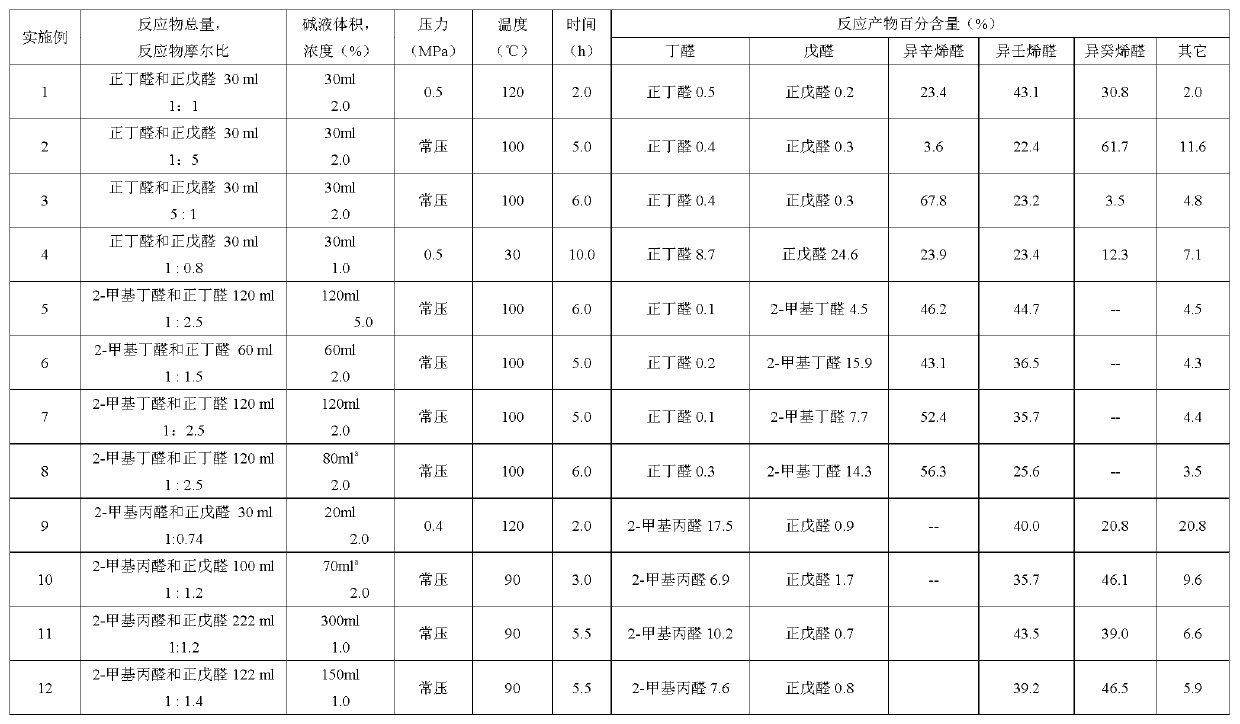
[0032] Add 30ml of n-butyraldehyde and n-valeraldehyde mixture and 30ml of 2% sodium hydroxide aqueous solution into the autoclave, wherein n-butyraldehyde: n-valeraldehyde=1:1 molar ratio; check the airtightness after closing the kettle to confirm that there is no air leakage , replace the air in the kettle with nitrogen for five times, and then pass nitrogen to pressurize to 0.5MPa, stir and heat up to 120°C, and react for 2 hours; after the reaction, cool to room temperature and reduce pressure, open the kettle, and take out the reaction The liquid is placed in a separatory funnel, and after phase separation, the upper organic phase is taken for analysis, and the lower lye is recovered; the reaction solution contains 0.5% of unreacted n-butyraldehyde, 0.2% of n-valeraldehyde, 23.4% of isooctenal, and iso-octenal. Nonenal 43.1%, isodecenal 30.8%; other components 2.0%.
Embodiment 2
[0034] In the three-necked flask, a spherical condenser, a thermometer and an inlet pipe are installed respectively; 30ml of n-butyraldehyde and n-valeraldehyde mixture, 2% sodium hydroxide aqueous solution 30ml are added in the three-necked flask, wherein n-butyraldehyde: n-valeraldehyde=1: 5 molar ratio; pass nitrogen into the device through the inlet pipe, replace the air in the device five times, and then fill in an appropriate amount of nitrogen to maintain the inert atmosphere in the device; place the three-necked flask on the oil bath; heat to 95 ~102°C, reaction time 5 hours; after the reaction, cool the reaction solution to room temperature, take out the reaction solution, take the upper organic phase for analysis after phase separation, and recover the lower lye; the reaction solution contains 0.4% n-butyraldehyde, n-butyraldehyde Valeraldehyde 0.3%, isoctenal 3.6%, isononenal 22.4%, isodecenal 61.7%; other components 11.6%.
Embodiment 3
[0036] In the three-necked flask, a spherical condenser, a thermometer and an inlet pipe are installed respectively; 30ml of n-butyraldehyde and n-valeraldehyde mixture, 2% sodium hydroxide aqueous solution 30ml are added in the three-necked flask, wherein n-butyraldehyde: n-valeraldehyde=5: 1 molar ratio; feed nitrogen into the device through the inlet pipe, replace the air in the device five times, and then fill in an appropriate amount of nitrogen to maintain the inert atmosphere in the device; place the three-necked flask on an oil bath; heat to 95 ~102°C, the reaction time is 6 hours; after the reaction, cool the reaction solution to room temperature, take out the reaction solution, take the upper organic phase for analysis after phase separation, and recover the lower lye; the reaction solution contains 0.4% n-butyraldehyde, n-butyraldehyde Valeraldehyde 0.3%, isoctenal 67.8%, isononenal 23.2%, isodecenal 3.5%, other components 4.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com