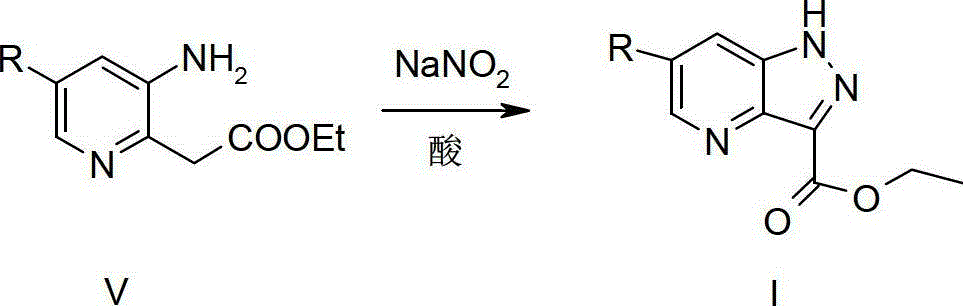
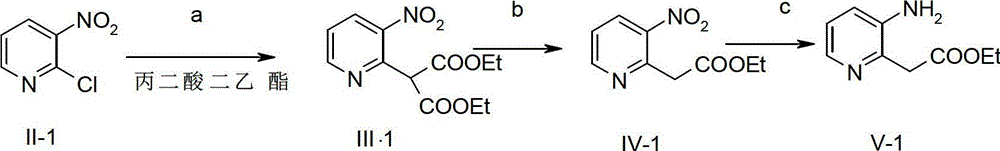Synthesis method of 1H-pyrazolo (4, 3-b) pyridine-3-carboxylic acid ethyl ester and 6-bromine-substituted compound thereof
A compound, the technology of ethyl acetoacetate, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of preparation without literature reports, and achieve the effects of increased yield, simplified post-processing, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of compound IV-1:
[0027]
[0028] A solution of 17% sodium ethoxide (EtONa) (514.7 g, 1.286 mol, 1.0 eq.) in ethanol (EtOH) was added to a 5 L four-necked flask, and 700 mL of EtOH was added. After cooling down in an ice bath, a solution of ethyl acetoacetate (167.5 g, 1.286 mol, 1.0 eq.) in EtOH (725 mL) was added dropwise to the reaction flask, and after the addition was complete, it was raised to room temperature and stirred for 30 min. Then, a solution of compound II-1 (204.25 g, 1.29 mol, 1.0 eq.) in THF (1250 mL) was added dropwise under ice-bath conditions. After the dropwise addition, the temperature was raised to reflux and reacted for 10 h. After the reaction was complete, the reaction solution was concentrated and poured into ice water. Extracted with EA, anhydrous Na 2 SO 4 The organic phase is dried. Concentrated under reduced pressure to obtain 227.7 g of compound IV-1 as a red oil, with a yield of 84.0%. 1 H NMR (400MHz, CDCl 3 ) δ (p...
Embodiment 2
[0036] Synthesis of compound IV-1:
[0037]
[0038] A solution of compound II-1 (204.25g, 1.29mol, 1.0eq.) in tetrahydrofuran (THF) (750mL) was added to a 5L four-necked flask, and NaH (60%) (128.4g, 3.22mol, 2.5eq. ), stirred at room temperature for 1 h after the addition, and added a THF (525 mL) solution of ethyl acetoacetate (418.7 g, 3.21 mol, 2.5 eq.) dropwise into the reaction flask. After the reaction was complete, the reaction solution was concentrated and poured into ice water. Extracted with EA, anhydrous Na 2 SO 4 The organic phase is dried. Concentration gave 248.1 g of compound IV-1 as a red oil, with a yield of 91.5%. 1 H NMR (400MHz, CDCl 3 ) δ (ppm) 8.80 (q, J = 1.52Hz, J = 3.20Hz, 1H), 8.44 (q, J = 1.52Hz, J = 6.76Hz, 1H), 7.48 (q, J = 4.76Hz, J = 3.52Hz, 1H), 4.34(s, 2H), 4.18-4.24(q, J=7.12Hz, 2H), 1.27(t, J=7.12Hz, 3H).
[0039] Synthesis of compound V-1:
[0040]
[0041] In a 2L single-necked bottle, 80.0g of compound IV-1 was completely d...
Embodiment 3
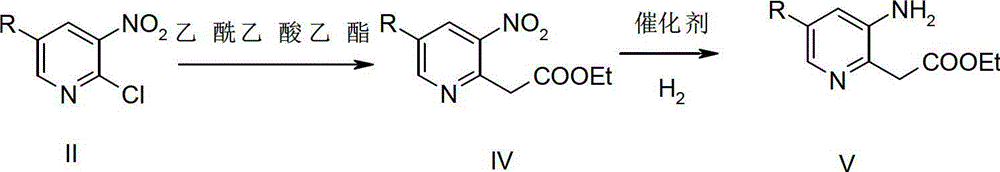
[0046] Synthesis of compound IV-2:
[0047]
[0048] Add 17% EtONa (51.47g, 0.1286mol, 1.0eq.) in EtOH solution to a 1L four-neck flask, and then add 70mL EtOH. After cooling down in an ice bath, a solution of ethyl acetoacetate (16.75 g, 0.1286 mol, 1.0 eq.) in EtOH (72.5 mL) was added dropwise to the reaction flask, and after the addition was complete, it was raised to room temperature and stirred for 30 min. Then, a solution of compound II-2 (30.63 g, 1.29 mol, 1.0 eq.) in THF (125 mL) was added dropwise under ice-bath conditions. After the dropwise addition, the temperature was raised to reflux and reacted for 10 h. After the reaction was complete, the reaction solution was concentrated and poured into ice water. Extracted with EA, anhydrous Na 2 SO 4 The organic phase is dried. Concentration under reduced pressure gave 31.32 g of compound IV-2 as a red oil, with a yield of 84.0%. 1 H NMR (400MHz, CDCl 3 ) δ (ppm) 8.86 (s, 1H), 8.04 (s, 1H), 8.59 (s, 1H), 4.30 (s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com