Chemical method for detecting 5-methylcystein in DNA
A technology of methylcytosine, chemical method, applied in the field of chemistry to detect 5-methylcytosine in DNA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
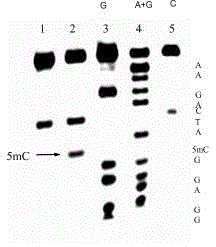
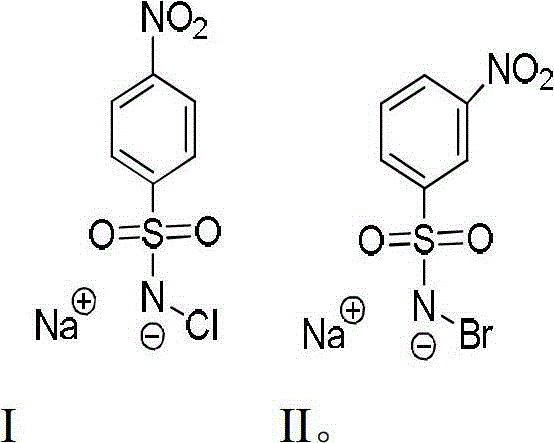
[0033] The invention adopts the DNA sequence fragment of the promoter region in gastric cancer and cervical cancer to analyze, after reacting with p-nitrobenzenesulfonyl chloride sodium salt and m-nitrobenzenesulfonyl bromide sodium salt, and then treating with piperidine , the position of 5-methylcytosine in the DNA sequence can be clearly seen.
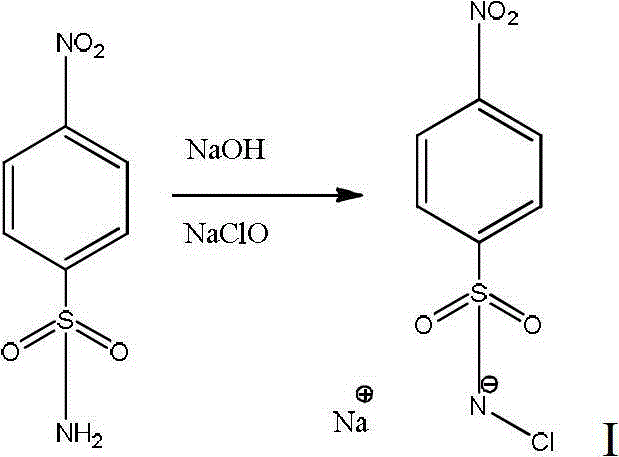
[0034] 1) Preparation of p-nitrobenzenesulfonyl chloride sodium salt
[0035] Add 0.8g of solid sodium hydroxide and 2mmol of p-nitrobenzenesulfonamide to 2ml of aqueous solution, stir for 5min, add 3ml of sodium hypochlorite solution, stir at room temperature for 12h, filter, wash the obtained solid with cold water for 5 times, and then reweight with water Crystallized to obtain pale yellow crystals. The yield was 83%. 1 H-NMR (300MHz, d 6 -DMSO)δ=8.27(d,J=7.5H z ,2H),7.88(d,J=7.5H z , 2H). 13 C-NMR (75MHz, d 6 -DMSO)δ=156.72,153.18,133.55,128.80.
[0036] 2) preparation of m-nitrobenzenesulfonyl bromide sodium salt
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com