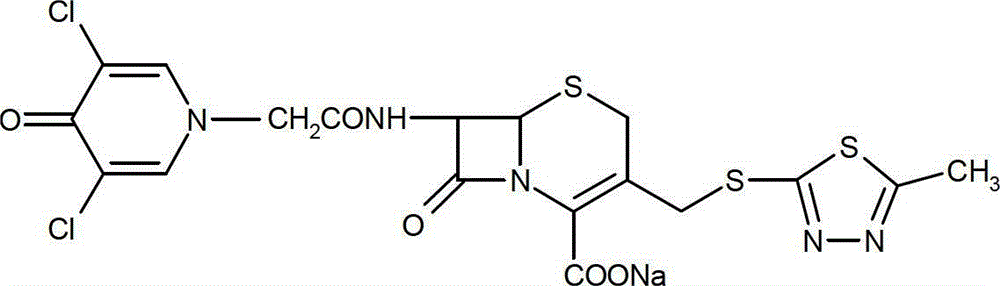
Method for determining moisture limit of cefazedone sodium sterile
A kind of technology of cefoxidone sodium and determination method, which is applied in the field of determination of water limit of cefoxidone sodium, and achieves the effects of ensuring stability and safety, and the method is simple and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The present invention will be further described in detail below through the specific examples, the following examples are only descriptive, not restrictive, and cannot limit the protection scope of the present invention with this.
[0038] A method for determining the water content control limit of cefazedone sodium, the steps are as follows:
[0039] 1. Sample
[0040] Cefazedone sodium for injection, batch number: 110304, 110305, product of Tianjin Xinfeng Pharmaceutical Co., Ltd. The raw materials are all cefzidone sodium produced by South Korea Shinfeng Pharmaceutical Co., Ltd.
[0041] 2. Measurement method
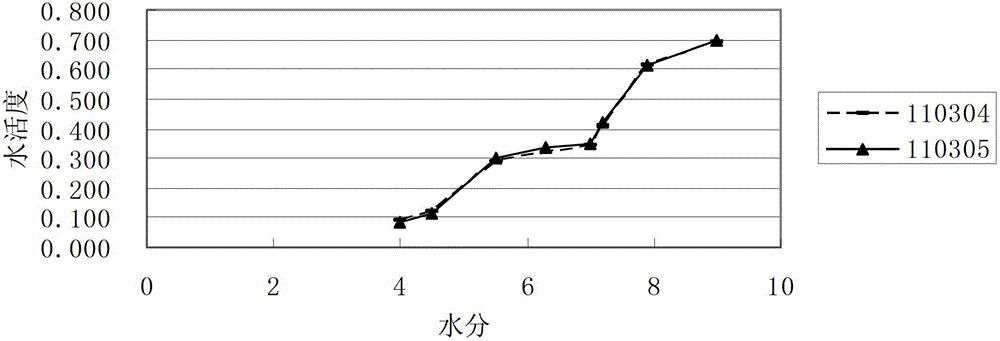
[0042] ⑴ Determination of water activity
[0043] Adopt AW SPRINT type water activity measuring instrument, the product of Swiss Novasina company; basic parameter setting: measuring temperature is 25 ℃; humidity stability factor is 2; calibration relative humidity points before testing are 3, respectively 11.3%RH, 52.9% RH, 75%RH; take a proper amount of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com