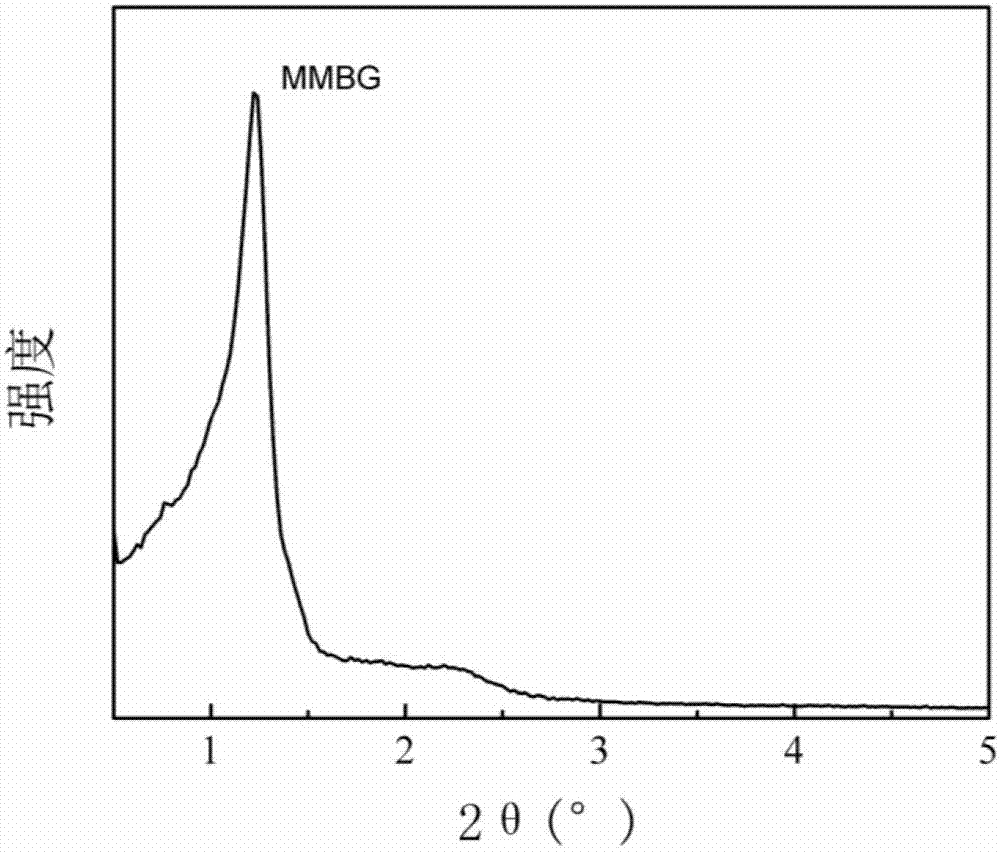
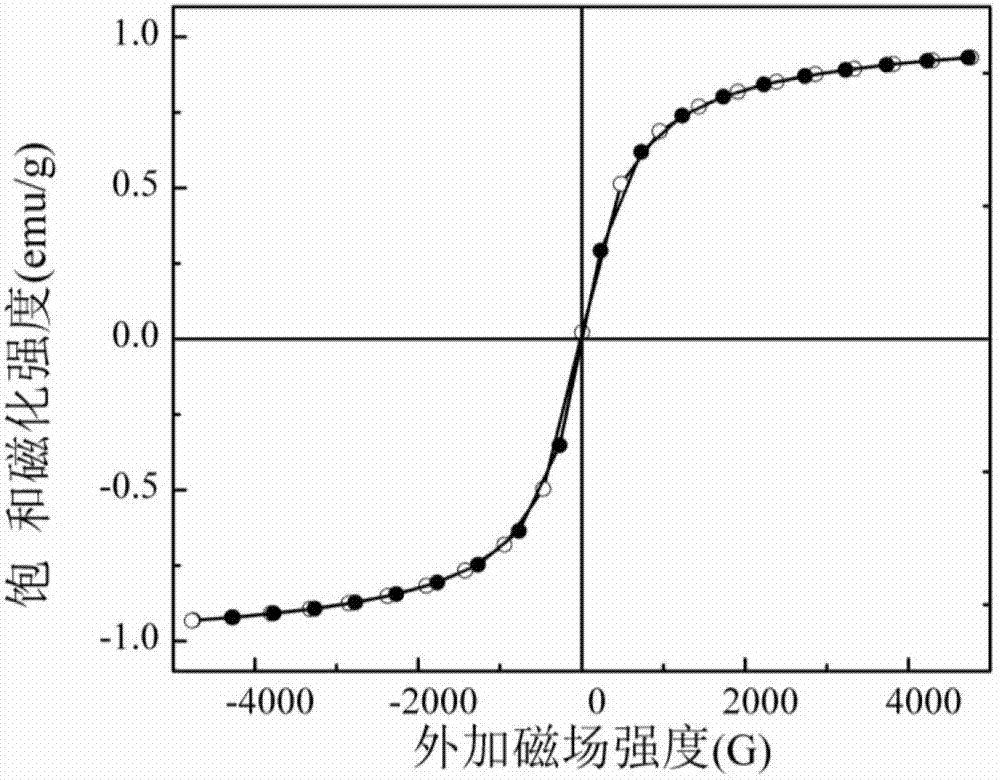
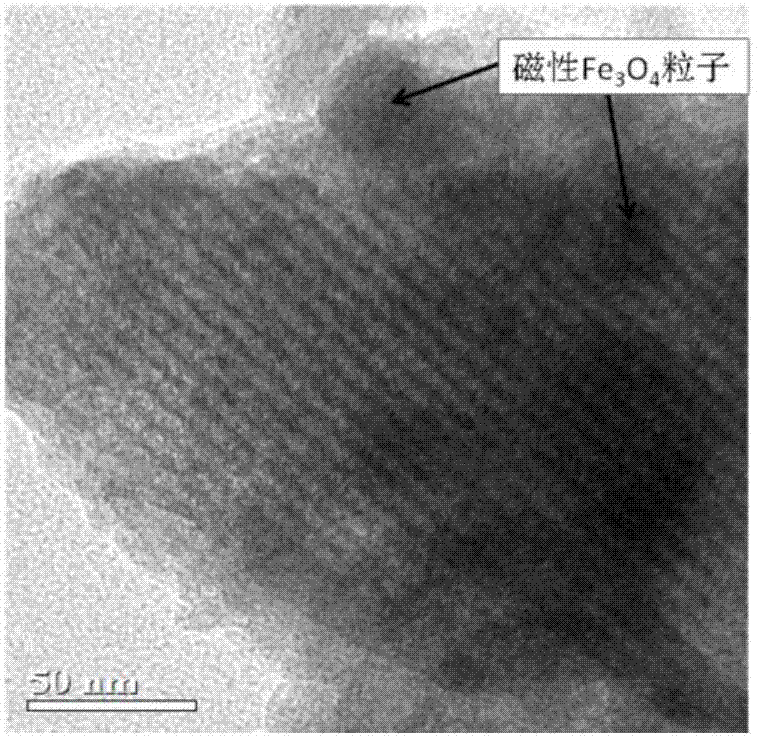
Magnetic mesoporous bioactive glass drug delivery system and preparation method thereof
A technology of biological glass and delivery system, applied in the fields of medical science, prosthesis, inorganic inactive ingredients, etc., can solve the problems of cytotoxicity and increase biocompatibility, and achieve increased drug loading, broad application prospects, and excellent drug loading. and sustained release properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0025] First place 4gP123 in 50mL of ethanol and stir vigorously to obtain a uniform surfactant ethanol dispersion solution; then prepare the inorganic precursor, in order to obtain the SiO as shown in Table 1 2 :CaO:P 2 o 5 :Fe 3 o 4 The mass ratio of the magnetic mesoporous bioglass drug delivery system, respectively adding inorganic substances in order, wherein the first group of embodiments specifically: 1.25g calcium nitrate tetrahydrate, 8.6mL (8g) tetraethyl orthosilicate (TEOS) , 0.35mL (0.37g) triethyl phosphate (TEP), 0.142g nano Fe 3 o 4 particles, then add 1-3 mL of dilute HNO 3 (1mol / L) solution is catalytically hydrolyzed at a reaction temperature of 40°C; after the inorganic precursor is hydrolyzed and dispersed evenly, the surfactant ethanol dispersion solution is added, and the reaction is fully mixed for soft template sealing and self-assembly into a sol-gel. Dry in an oven at ℃ for 1-3 days, and calcinate the gel obtained after aging at 550-700℃ for 5-...
Embodiment 7
[0029] According to the steps in Example 1, no magnetic nanoparticles were added, other processes and conditions were the same as in Example 1, and finally a non-magnetic mesoporous bioglass drug delivery system was obtained, which was the blank control group.
Embodiment 8
[0031]The magnetic mesoporous bioglass drug delivery system prepared in Example 1 was selected for drug loading and sustained release experiments.
[0032] (1) Drug loading: Weigh 30mg powder gentamicin sulfate in the glove box under the protection of inert gas, dissolve it in 50mL deionized water to make a 600μg / mL gentamicin sulfate solution; take 0.5g sample and put it in the solution , under constant temperature and vigorous stirring at 37°C for 24h. Centrifuge at 8000r / min, dry the lower precipitate in a vacuum oven at 37°C, and keep the upper clear for drug loading test.
[0033] (2) Drug release: Weigh 0.2 g of the drug-loaded sample, press it into a sheet with a diameter of 2-3 cm with a tablet machine, soak the sample in 100 mL of deionized water, and use UV- A visible spectrophotometer detects the drug concentration in solution. Its drug release profile is as follows Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com