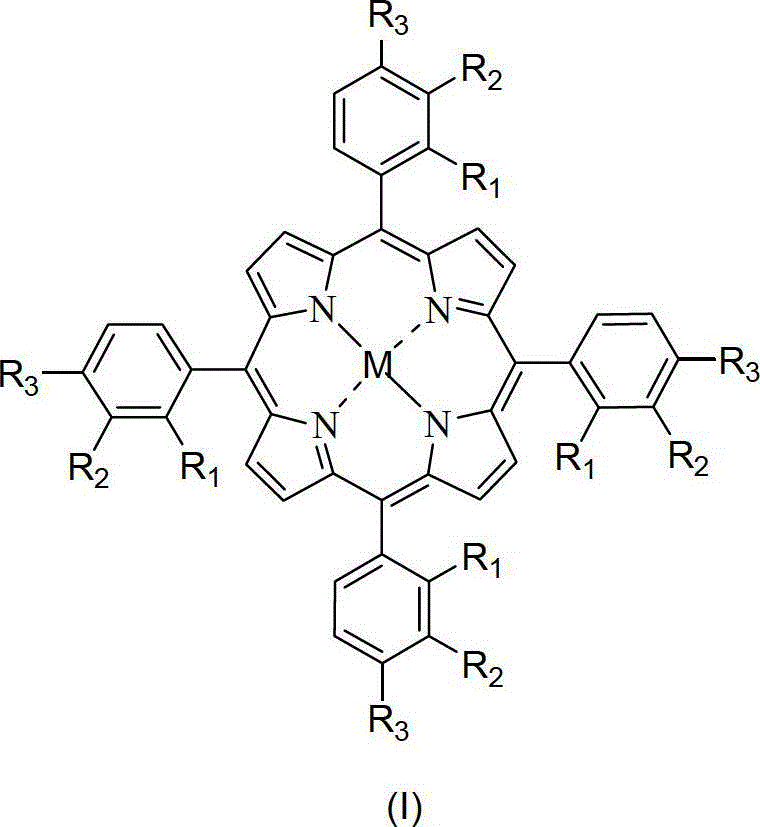
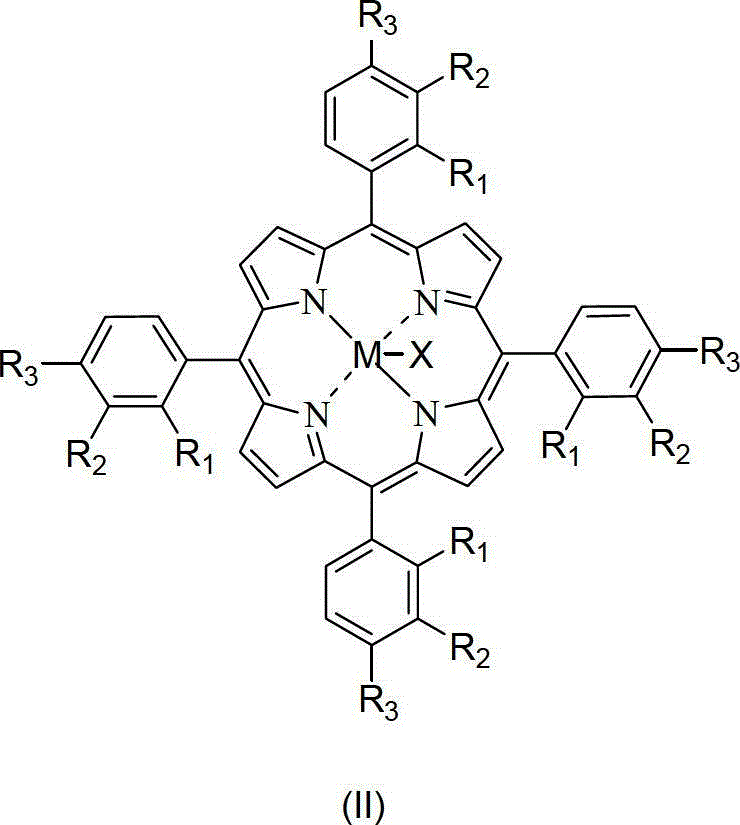
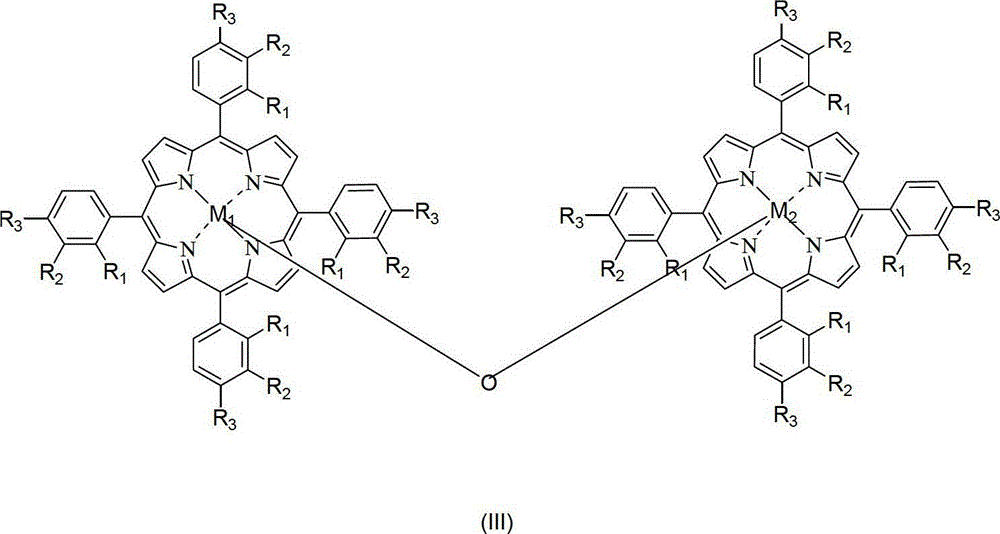
Catalytic system for preparing cyclohexanol and cyclohexanone by liquid-phase catalytic oxidation of cyclohexane and use method of catalytic system
A technology of liquid-phase catalysis and catalytic system, which is applied in the direction of hydrocarbon oxidation to prepare oxygenates, oxidation reaction preparation, chemical instruments and methods, etc. It can solve the problems of high purity requirements of metalloporphyrins and low selectivity of KA oil, and achieve the goal of synthesizing The price is cheap, the effect of reducing the problem of refining and purification, and reducing the cost of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] In a 1.2L batch oxidation reactor, the 18mg metalloporphyrin (R 1 =R 2 =H,R 3 =Cl, M=Ru) and 12g of cyclohexanol were added to 600g of cyclohexane, 10atm of air was introduced, and the reactant was stirred at 150°C. After about 90 minutes, the reaction was initiated, and the tail oxygen began to drop significantly. The induction period ended, and the reaction was stopped after continuing to ventilate the air for 0.7 hours. The reactant was rapidly cooled and then sampled for analysis. Analyze the selectivity of cyclohexanol, cyclohexanone, cyclohexyl hydroperoxide and other by-products generated in the reaction cooling liquid and calculate the conversion rate of cyclohexane. The reaction results are shown in Table 1.
Embodiment 2
[0057] In a 1.2L batch oxidation reactor, the metalloporphyrin (R 1 =R 2 =H,R 3 =Cl, M=Ru) and 12g of cyclohexanol were added to 600g of cyclohexane, 10atm of air was introduced, and the reactant was stirred at 150°C. After about 90 minutes, the reaction was initiated, and the tail oxygen began to drop significantly. The induction period ended, and the reaction was stopped after continuing to ventilate the air for 0.7 hours. The reactant was rapidly cooled and then sampled for analysis. Analyze the selectivity of cyclohexanol, cyclohexanone, cyclohexyl hydroperoxide and other by-products generated in the reaction cooling liquid and calculate the conversion rate of cyclohexane. The reaction results are shown in Table 1.
Embodiment 3
[0059] In a 1.2L batch oxidation reactor, the metalloporphyrin (R 1 =R 2 =H,R 3 =Cl, M=Ru) and 12g of cyclohexanol were added to 600g of cyclohexane, 10atm of air was introduced, and the reactant was stirred at 150°C. After about 90 minutes, the reaction was initiated, and the tail oxygen began to drop significantly. The induction period ended, and the reaction was stopped after continuing to ventilate the air for 0.7 hours. The reactant was rapidly cooled and then sampled for analysis. Analyze the selectivity of cyclohexanol, cyclohexanone, cyclohexyl hydroperoxide and other by-products generated in the reaction cooling liquid and calculate the conversion rate of cyclohexane. The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com