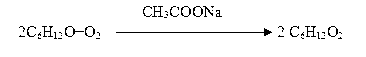Production process of matricaria ester
A production process, the technology of mamethrin, applied in the chemical field, can solve the problems of low utilization rate of raw materials, and achieve the effects of small investment, reduced production cost, and simple and uncomplicated production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] One, the preparation technology of 2-methylpentanoic acid is:
[0039] (1) Feeding: Feed 160 kg of 2-methylpentanal and 1.6 kg of anhydrous sodium acetate, and then feed the air;
[0040] (2) Oxidation reaction: control temperature: 10-30°C, reaction time: 24 hours, air velocity 2.0-3.0m 3 / h;
[0041] (3) Cooling: cooling temperature: internal temperature ≤ 20 ℃;
[0042] (4) Vacuum fractionation: refraction measurement per hour: below 1.410 is the head, above 1.410 is the finished product, and the finished product meets the Q / 320583XYP019-2008 standard.
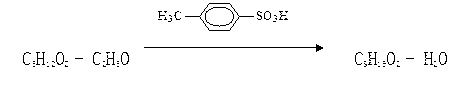
[0043] Two, the preparation technology of ethyl 2-methylpentanoate is:
[0044] (1) Feeding: Feed 250 kg of 2-methylpentanoic acid, 110 kg of ethanol and 2.5 kg of p-toluenesulfonic acid;
[0045] (2) Esterification reaction: control 110°C-130°C for 14 hours;
[0046] (3) Cooling: cooling temperature: internal temperature ≤ 40 ℃;
[0047] (4) Washing:
[0048] Inhale the crude product generated by the esterifi...
Embodiment 2
[0051] One, the preparation technology of 2-methylpentanoic acid is:
[0052] (1) Feeding: Feed 80 kg of 2-methylpentanal and 0.8 kg of anhydrous sodium acetate, and then feed the air;
[0053] (2) Oxidation reaction: control temperature: 0-15°C, reaction time: 25 hours, air velocity 1.5-2.0m 3 / h;
[0054] (3) Cooling: cooling temperature: internal temperature ≤ 20 ℃;
[0055] (4) Vacuum fractionation: refraction measurement per hour: below 1.410 is the head, above 1.410 is the finished product, and the finished product meets the Q / 320583XYP019-2008 standard.
[0056] Two, the preparation technology of ethyl 2-methylpentanoate is:
[0057] (1) Feeding: Feed 500 kg of 2-methylpentanoic acid, 220 kg of ethanol and 5 kg of p-toluenesulfonic acid;
[0058] (2) Esterification reaction: control 95°C-110°C for 12 hours;
[0059] (3) Cooling: cooling temperature: internal temperature ≤ 40 ℃;
[0060] (4) Washing:
[0061] Inhale the crude product generated by the esterification ...
Embodiment 3
[0064] One, the preparation technology of 2-methylpentanoic acid is:
[0065] (1) Feeding: Feed 200 kg of 2-methylpentanal and 2 kg of anhydrous sodium acetate, and then feed the air;
[0066] (2) Oxidation reaction: control temperature: 15°C, reaction time: 30 hours, air velocity 2.0m 3 / h;
[0067] (3) Cooling: cooling temperature: internal temperature ≤ 20 ℃;
[0068] (4) Vacuum fractionation: refraction measurement per hour: below 1.410 is the head, above 1.410 is the finished product, and the finished product meets the Q / 320583XYP019-2008 standard.
[0069] Two, the preparation technology of ethyl 2-methylpentanoate is:
[0070] (1) Feeding: Feed 1000 kg of 2-methylpentanoic acid, 440 kg of ethanol and 10 kg of toluenesulfonic acid;
[0071] (2) Esterification reaction: controlled at 110°C for 15 hours;
[0072] (3) Cooling: cooling temperature: internal temperature ≤ 40 ℃;
[0073] (4) Washing:
[0074] Inhale the crude product generated by the esterification reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com