Sox9 inhibitors
A technology of inhibitors and pharmaceuticals, applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, cyclic peptide ingredients, etc., can solve problems such as therapies that do not promote CNS damage or disease regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Astrocyte Culture System
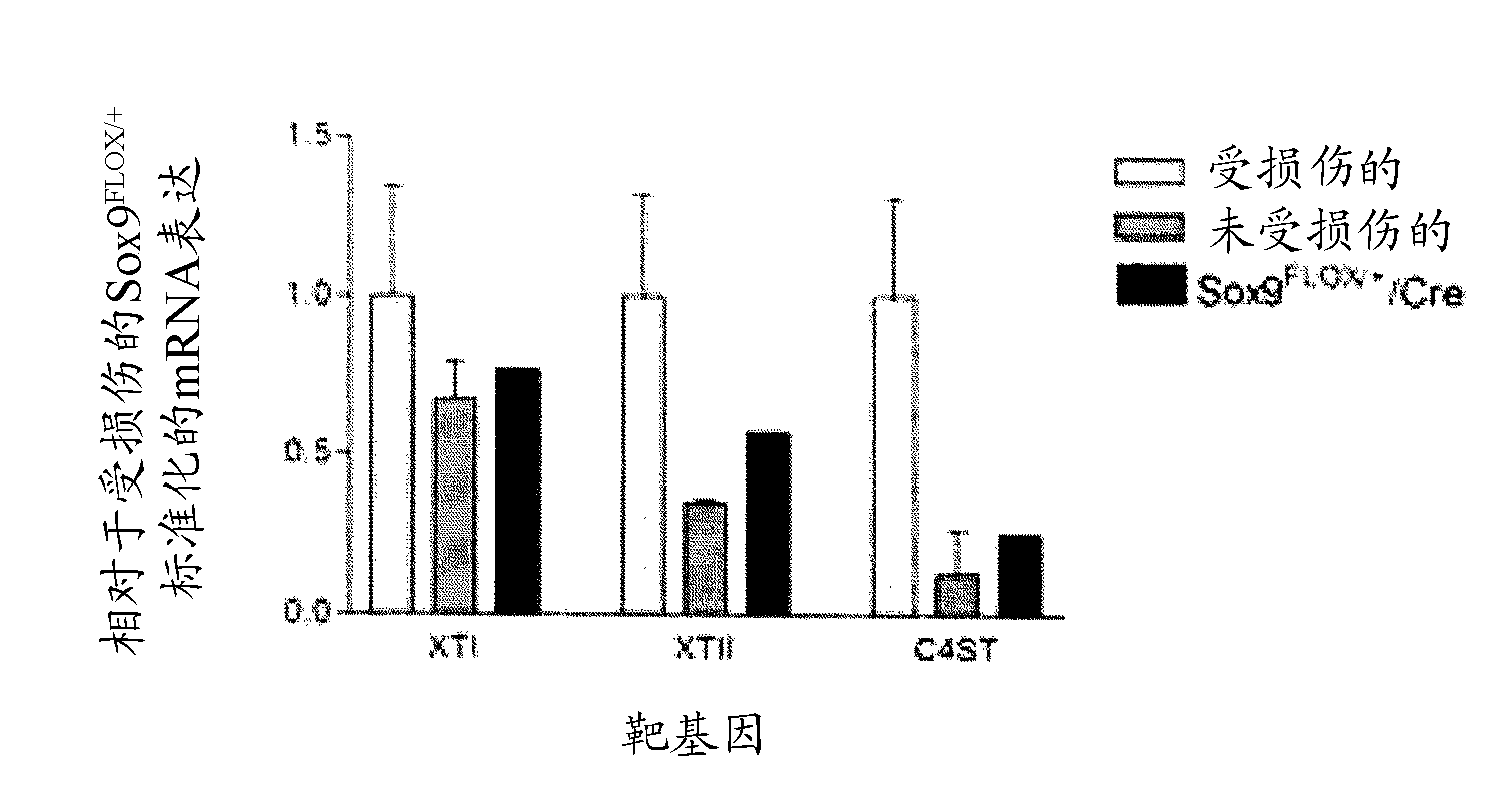
[0066] It has been established that the transcription factor SOX9 is able to upregulate the transcription of XT-I, XT-II and C4ST and downregulate the expression of the pro-regenerative extracellular matrix (ECM) molecules laminin and fibronectin in primary astrocyte cultures . Consistent with the assumption that CSPG genes are jointly regulated, Genomatix software analysis identified 5 transcription factors with putative binding sites in all 9 of their respective promoters. The relative positioning and characteristics of these units are shown in figure 1 middle.
[0067] To determine whether SOX9 upregulated the expression of XT-I, XT-II, and C4ST was direct or indirect, chromatin immunoprecipitation (ChIP) analysis was performed. Chromatin immunoprecipitation (ChIP) using anti-SOX9 antibodies on cells from the germline ridges of female (non-SOX9 expressing) or male (SOX9 expressing) mice confirmed that SOX9 binds to the promote...
Embodiment 2-S
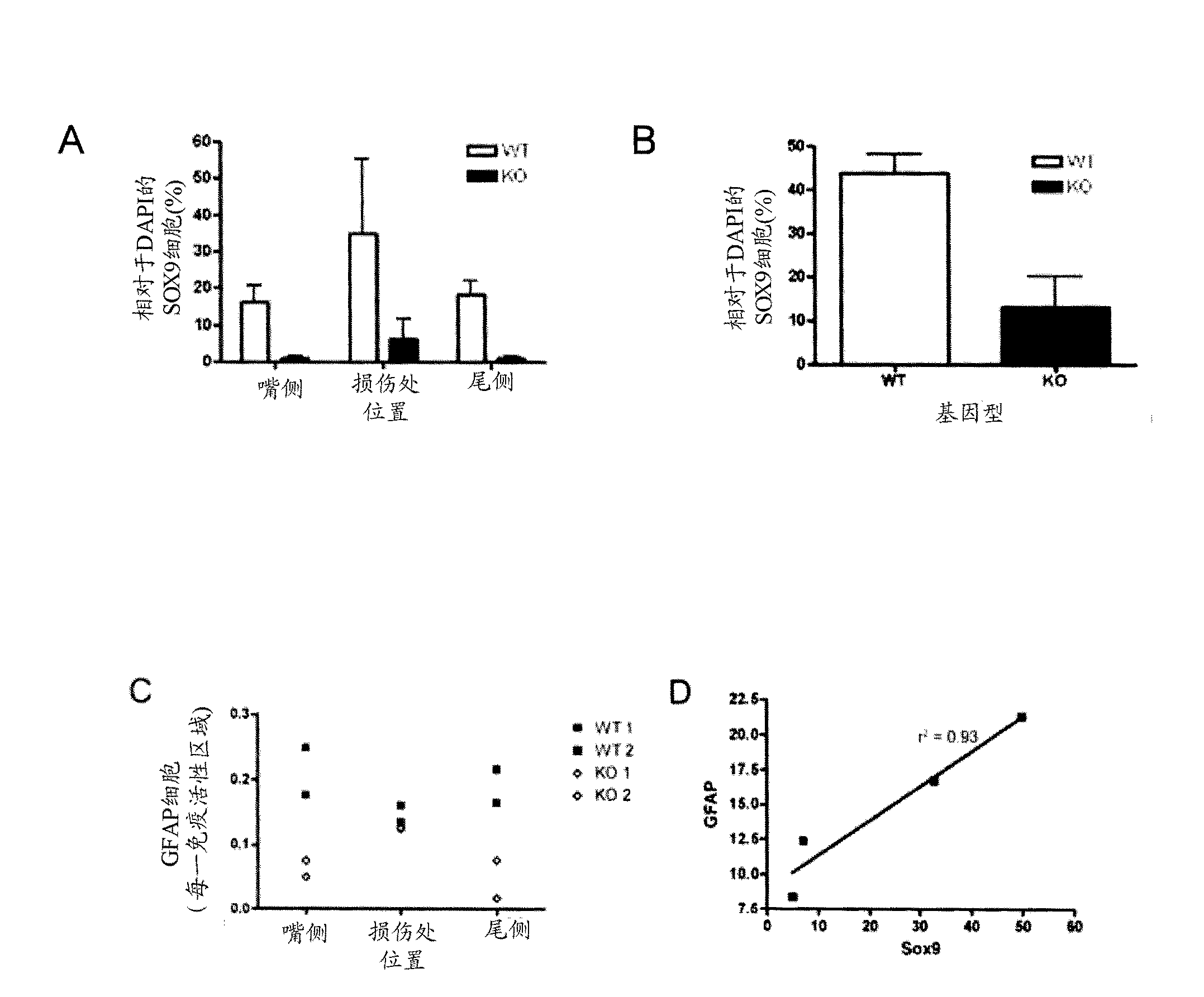
[0070] Example 2-Sox9 is associated with various CNS disorders
[0071] To evaluate the potential role of SOX9 in human CNS injury and disease, the expression of SOX9 in human hemorrhagic stroke (n=3), ischemic stroke (n=3), traumatic brain injury (n=2) cases was investigated . Briefly, human tissues were formalin-fixed and either frozen-sectioned or paraffin-embedded and sectioned for histological examination. Sections were stained with a combination of commonly commercially available differentially fluorescent antibodies, including GFAP, CS56, and Sox9, followed by counterstaining with the fluorescent nuclear dye DAPI. SOX9 expression was clearly observed in GFAP-positive astrocytes and in CSPG-enriched regions in samples from human tissues following ischemic stroke, TBI, and spinal cord injury. Uninjured human CNS samples were devoid of Sox9, GFAP and CSPG staining. Similarly, immunohistochemical staining of mouse tissues after MCAO and SCI showed a pattern similar to th...
Embodiment 3
[0081] Example 3 - Calmodulin antagonists affect SOX9 expression in astrocytes and SCI
[0082] The efficacy of calmodulin antagonists (inhibitors) to reduce the expression of SOX9 target genes was evaluated in cultured rat astrocytes transfected with pGL4.1 4x48 Col2a1 as described in Example Prepared as described in 1. This plasmid contains a 4-48bp SOX9 binding site from the Col2A1 enhancer, which promotes expression of the luciferase reporter gene in cells where SOX9 is active in the nucleus. The next day after transfection, the cells were used as follows and in Figure 5 Inhibitors were treated for 24 h at the concentrations indicated in the luciferase assay. In the case of chlorpromazine, there was a clear dose-dependent response limiting luciferase expression in mature astrocyte cultures, optimized to be most pronounced at 20 μM ( Figure 5 A, B, D). In addition, the calmodulin inhibitors W7 and W5 also showed a dose-dependent decrease in reporter activity, showing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com