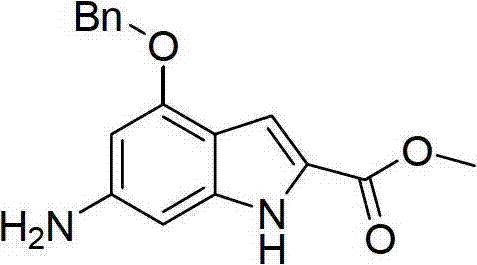
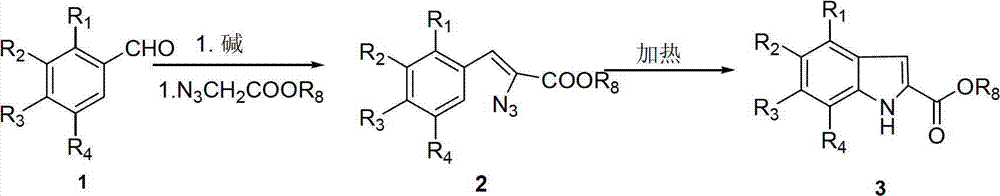
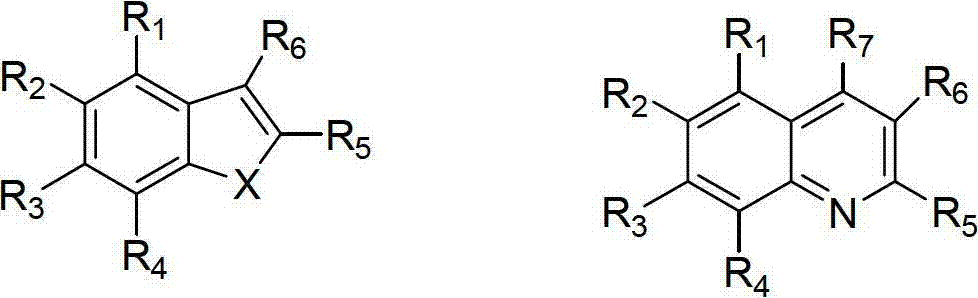Substituted benzoheterocyclic compounds and preparation method and application thereof
A compound and unsubstituted technology, applied in the field of substituted benzoheterocyclic compounds and their synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0223] (Z)-2-azido-3-(4-acetamidophenyl) methyl acrylate (2a)
[0224] 4-Acetaminobenzaldehyde (2g, 0.0122mol) was dissolved in methanol (30ml), sodium methoxide (0.85g, 0.0159mol) was added, and methyl azide acetate in methanol (1.65g, 0.0128mol) was added dropwise at room temperature, After dropping, react at room temperature for 1.5h. After the reaction was completed, the reaction solution was added to saturated ammonium chloride solution, extracted with ethyl acetate (3×50ml), and the organic layers were combined. Wash the organic layer with saturated brine, anhydrous MgSO 4 Dry overnight. After filtration, the filtrate was evaporated under reduced pressure to remove the solvent, and the residue was passed through a column to obtain a pale yellow solid (2.7 g, 85%). Because the compound is unstable, it has not been characterized by NMR.
Embodiment 2
[0226] Methyl 6-acetylaminoindole-2-carboxylate (3a)
[0227] Compound 2a (2.7g, 0.0104mol) was dissolved in chlorobenzene (50ml) and heated to reflux for 2.5h. After the reaction was completed, a white solid precipitated out after cooling, and the target product was obtained by filtration, a white solid (2.08 g, 86%). 1 H-NMR(400M,DMSO-d 6 )δ(ppm): 2.05(s, 3H), 3.83(s, 3H), 7.07(s, 1H), 7.10(d, J=8.8Hz, 1H), 7.52(d, J=8.8Hz, 1H) ,8.01(s,1H),9.96(s,1H),11.75(s,1H).
Embodiment 3
[0229] Methyl 6-aminoindole-2-carboxylate (3b)
[0230] Compound 3a (1g, 4.31mmol) was dissolved in a methanol solution of hydrogen chloride, and the mixture was heated to reflux for 2h. After the reaction was completed, the solvent was evaporated under pressure, and the residue was recrystallized from anhydrous methanol to obtain a white solid (0.37 g, 78%). 1 H-NMR(400M,DMSO-d 6 )δ(ppm): 3.87(s,3H), 6.94(d,J=8.4Hz,1H), 7.16(s,1H), 7.32(s,1H), 7.68(d,J=8.4Hz,1H) ,9.49(br,2H),11.99(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com