Clean production process of dicamba synthesis midbody
A clean production and chemical synthesis technology, applied in the preparation of organic compounds, organic chemistry, chemical instruments and methods, etc., can solve problems such as difficult to fully react, environmental impact, etc., and achieve the effect of increasing reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
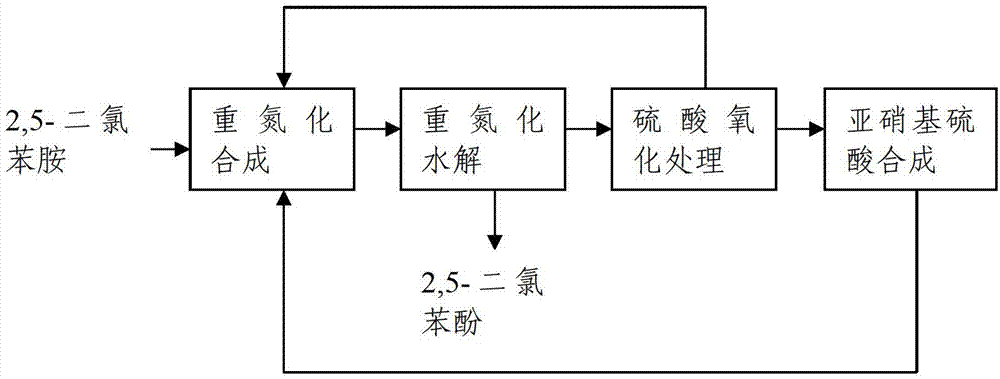
[0021] Synthesis of diazotization: clean production process of 2,5-dichlorophenol synthesis in the production of dicamba, first stir 90g of 2,5-dichloroaniline and 540g of 60% dilute sulfuric acid under reflux for 2 hours, and cool the reaction solution To 0-5°C, add 187.5g of 40% nitrosylsulfuric acid, stir for 1.5 hours, control the molar ratio of 2,5-dichloroaniline at 1:1.05, and pass the diazonium solution generated by the reaction with nitrosylsulfuric acid 60%-70% sulfuric acid is hydrolyzed at a high temperature of 140°C, and the obtained 2,5-dichlorophenol gas is condensed and separated, and then centrifuged and dehydrated to obtain a solid product. Part of the waste sulfuric acid obtained from hydrolysis is recovered by high vacuum distillation and directly applied to diazotization to synthesize dilute sulfuric acid, and the other part is mixed with SO produced by other processes of the company. 2 Tail gas synthesis of 40% nitrosyl sulfuric acid is then applied to di...
Embodiment 2
[0025] Diazotization synthesis: 2,5-dichlorophenol synthesis clean production process in the production of dicamba, first stir 90g of 2,5-dichloroaniline and 720g of 50% dilute sulfuric acid under reflux for 2 hours, then cool the reaction solution To 0-5 ℃, put in 393g of 20% nitrosyl sulfuric acid, stir for 2 hours, the molar ratio of 2,5-dichloroaniline is controlled at 1:1.1, and the diazonium solution generated by the reaction with nitrosyl sulfuric acid is passed into 60%-70% sulfuric acid is hydrolyzed at a high temperature of 150°C, and the obtained 2,5-dichlorophenol gas is condensed and separated, and then centrifuged and dehydrated to obtain a solid product. The dilute sulfuric acid obtained by hydrolysis is oxidized and recovered by 35% hydrogen peroxide, and part of it is directly applied and diazotized to form dilute sulfuric acid, and the other part is mixed with SO produced by other processes of the company. 2 Tail gas synthesis of 20% nitrosyl sulfuric acid is...
Embodiment 3
[0029] Diazotization synthesis: 2,5-dichlorophenol synthesis clean production process in dicamba production, first stir 90g of 2,5-dichloroaniline and 810g of 40% dilute sulfuric acid under reflux for 2 hours, then cool the reaction solution To 0-5°C, add 142.3g of 60% nitrosyl sulfuric acid and stir for 2 hours. The molar ratio of 2,5-dichloroaniline is controlled at 1:1.2. %-70% sulfuric acid is hydrolyzed at a high temperature of 170°C, and the obtained 2,5-dichlorophenol gas is condensed and separated, and then centrifuged to dehydrate to obtain a solid product. The dilute sulfuric acid obtained by hydrolysis is extracted and recovered with chloroform, and part of it is directly applied mechanically and diazotized to synthesize dilute sulfuric acid, and the other part is mixed with SO produced by other processes of the company. 2 Tail gas synthesis of 60% nitrosyl sulfuric acid is then applied to diazotization synthesis, and the yield of 2,5-dichlorophenol in this example ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com