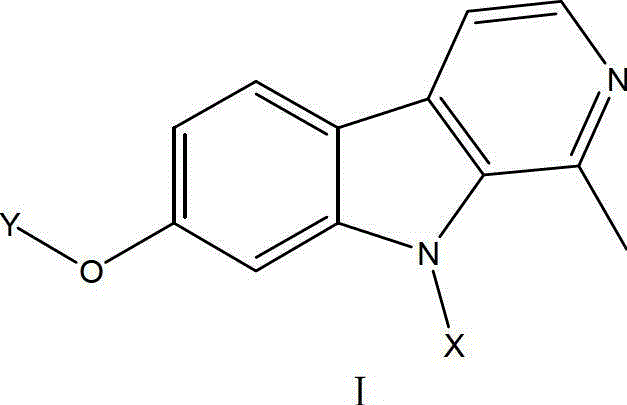
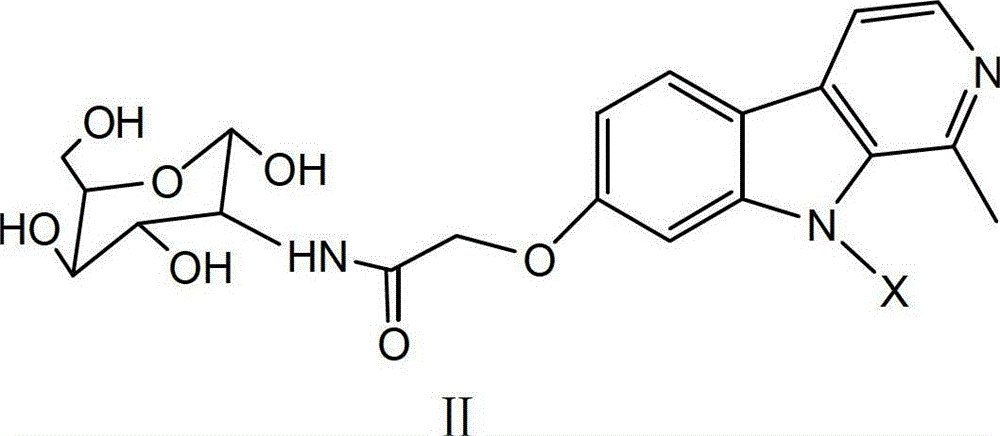
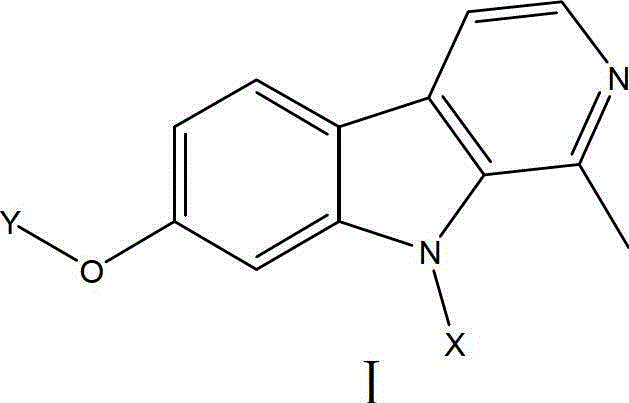
Harmine derivative anti-tumor prodrug with target characteristic
A technology of dehydrohalamine and its derivatives, which is applied in the fields of antineoplastic drugs, sugar derivatives, sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of I-1:
[0038] Step A: Mix harmine (1.06g, 5mmol), N,N-dimethylformamide (25ml), sodium hydrogen (0.192g, 8mmol), stir at room temperature for 10min, add 3-phenylpropyl bromide ( 10mmol), stirring for 0.5-3h, TLC tracking detection (petroleum ether / acetone=1:1), the reaction is completed, the reaction mixture solution is poured into 50ml ice water, stirred for 30min, filtered, and washed with a lot of water. The solid was dissolved in absolute ethanol, adjusted to pH 3-4 with hydrochloric acid, and concentrated to dryness under reduced pressure. The absolute ethanol was carried with water several times to obtain an oil, which was recrystallized with acetone to obtain white crystals as the hydrochloride salt of the product.
[0039] ESI-MS m / e(M+1)331.2
[0040] 1 H-NMR (500MHz, CDCl 3 )δ8.23-8.24 (1H, d, J=5.1Hz); 7.81-7.82 (1H, d, J=5.13Hz); 8.10-8.08 (1H, d, J=8.6Hz); 7.11-7.12 (1H , M); 6.86-6.88 (1H, m); 7.11-7.30 (5H, m); 3.90 (3H, s); 3.15 (3H, s); 2.01-2...
Embodiment 2
[0048] Preparation of I-2:
[0049] Step A: The preparation method is the same as in Example 1. In the step, bromoethane is used as the alkylating agent.
[0050] ESI-MS m / e(M+1)241.3
[0051] 1 H-NMR (500MHz, CDCl 3 )δ8.23-8.24 (1H, d, J=5.1Hz); 7.81-7.82 (1H, d, J=5.13Hz); 8.10-8.08 (1H, d, J=8.6Hz); 7.11-7.12 (1H , M); 6.86-6.88 (1H, m); 3.90 (3H, s); 3.15 (3H, s); 4.46-4.53 (2H, m); 1.29-1.34 (3H, m)
[0052] Step B: The preparation method is the same as in Example 1.
[0053] ESI-MS m / e(M+1)227.2
[0054] 1 H-NMR (500MHz, CDCl 3 )δ9.70 (1H, s, OH); 8.23-8.24 (1H, d, J = 5.1 Hz); 7.81-7.82 (1H, d, J = 5.13 Hz); 8.10-8.08 (1H, d, J = 8.6Hz); 7.11-7.12 (1H, m); 6.86-6.88 (1H, m); 3.15 (3H, s); 4.46-4.53 (2H, m); 1.29-1.34 (3H, m)
[0055] Step C: The preparation method is the same as in Example 1.
[0056] ESI-MS m / e(M+1)313.3
[0057] 1 H-NMR (500MHz, CDCl 3 )δ8.23-8.24 (1H, d, J=5.1Hz); 7.81-7.82 (1H, d, J=5.13Hz); 8.10-8.08 (1H, d, J=8.6Hz); 7.11-7.12 (1H , M); 6.86-6.88 (1H, m); 3....
Embodiment 3
[0059] Preparation of I-3:
[0060] Step A: The preparation method is the same as in Example 1. In the step, bromobutane is used as the alkylating agent.
[0061] ESI-MS m / e(M+1)269.3
[0062] 1 H-NMR (500MHz, CDCl 3 )δ8.23-8.24(1H, d, J=5.1Hz); 7.81-7.82(1H, d, J=5.13Hz); 8.10-8.08(1H, d, J=8.6Hz); 7.11-7.12(1H , M); 6.86-6.88 (1H, m); 3.90 (3H, s); 3.15 (3H, s); 3.84-3.86 (2H, m); 1.76-1.78 (2H, m); 1.32-1.34 (2H , M); 0.95-0.97 (3H, m)
[0063] Step B: The preparation method is the same as in Example 1.
[0064] ESI-MS m / e(M+1)255.7
[0065] 1 H-NMR (500MHz, CDCl 3 )δ9.74 (1H, s, OH); 8.23-8.24 (1H, d, J=5.1Hz); 7.81-7.82 (1H, d, J=5.13Hz); 8.10-8.08 (1H, d, J= 8.6Hz); 7.11-7.12 (1H, m); 6.86-6.88 (1H, m); 3.15 (3H, s); 3.84-3.86 (2H, m); 1.76-1.78 (2H, m); 1.32-1.34 (2H, m); 0.95-0.97 (3H, m)
[0066] Step C: The preparation method is the same as in Example 1.
[0067] ESI-MS m / e(M+1)341.3
[0068] 1 H-NMR (500MHz, CDCl 3 )δ8.23-8.24 (1H, d, J=5.1Hz); 7.81-7.82 (1H, d, J=5.13Hz); 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com